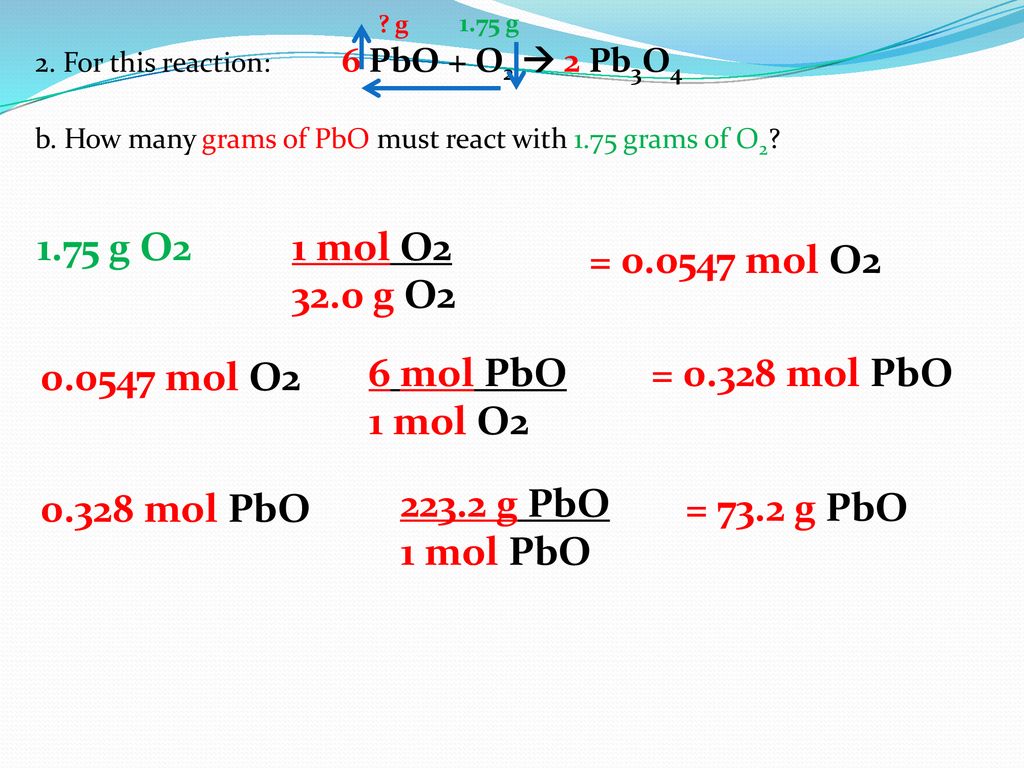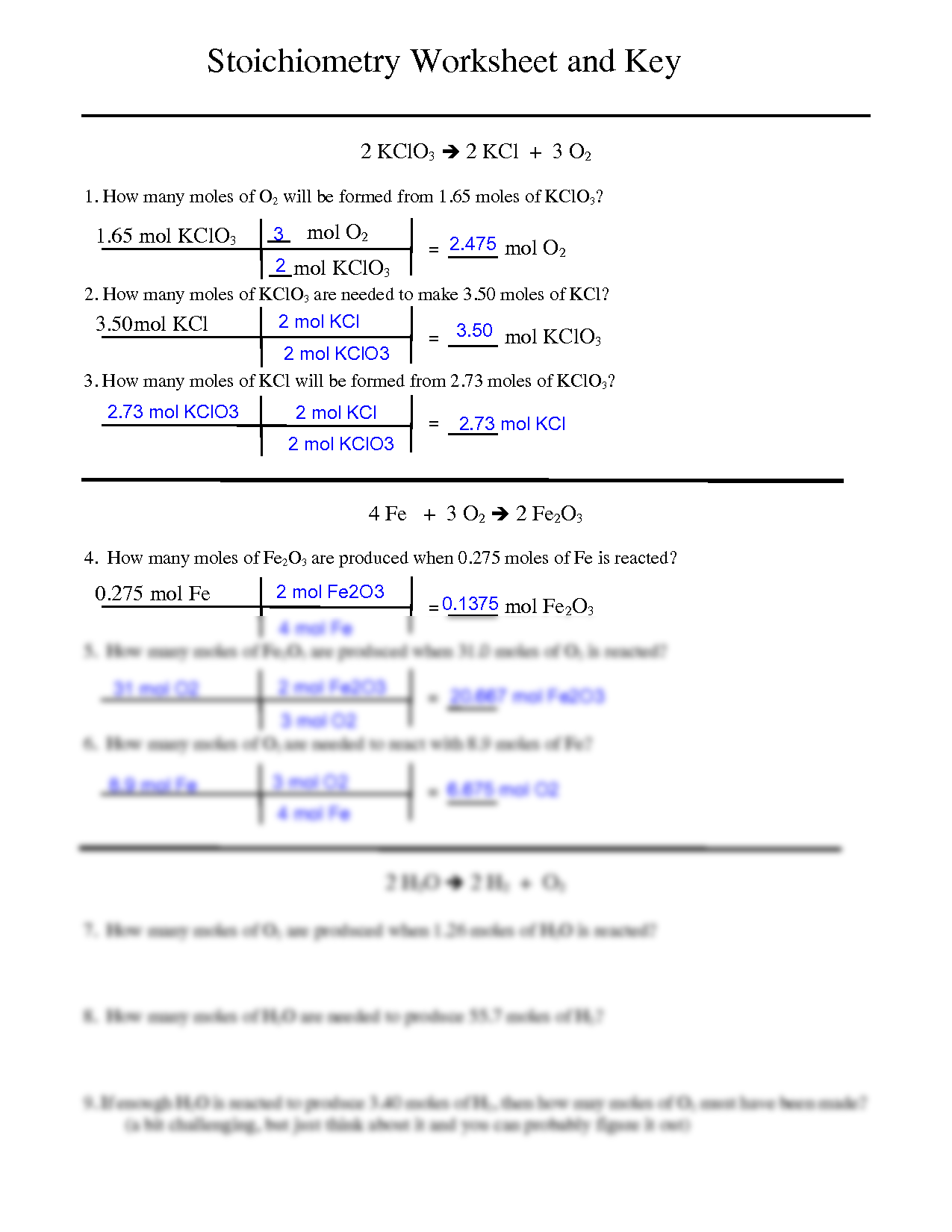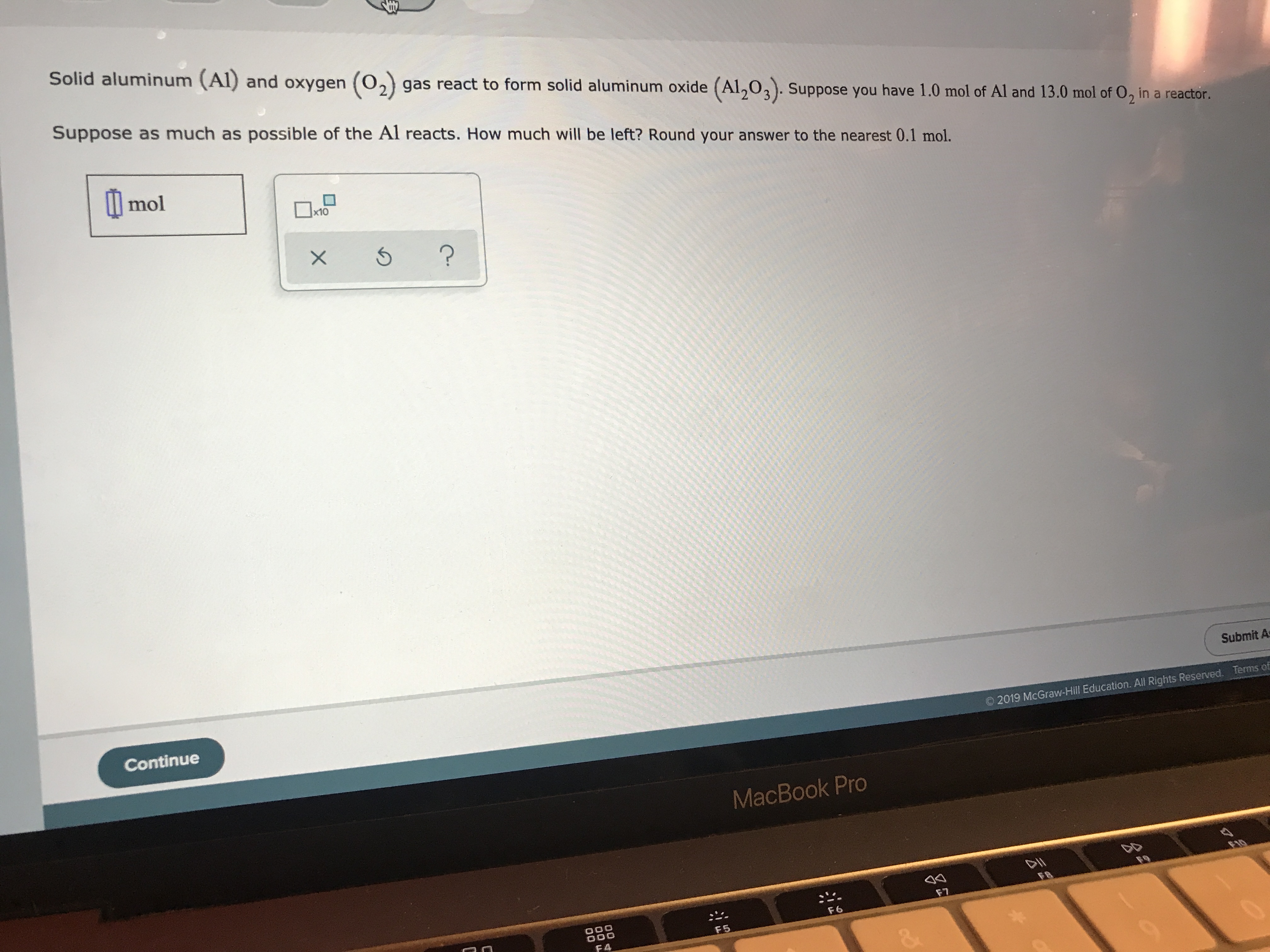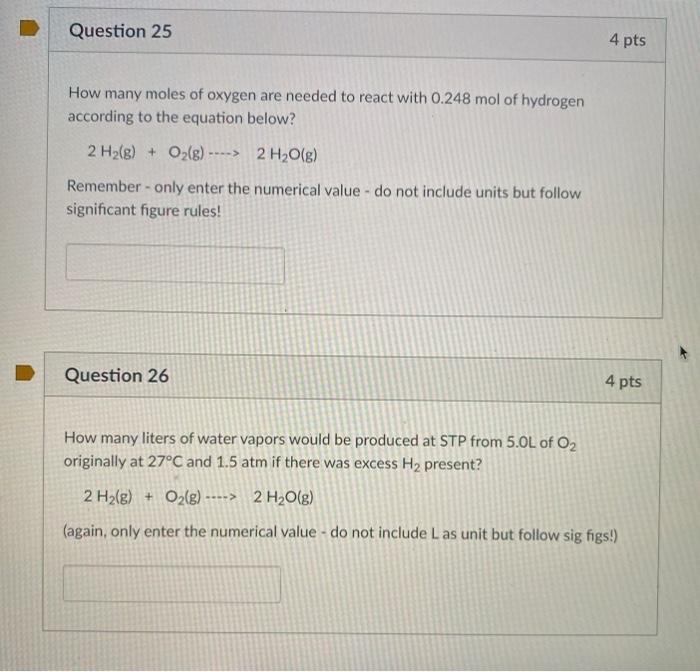
SOLVED: What mass of oxygen combines with 2.6 mol of magnesium in the reaction 2Mg + O2 = 2MgO? Answer in units of g.

SOLVED: How many molecules are there in 0.50 mol of O2? a. 1.2x10^25 b. 6.0x10^23 c. 2 d. 1 e. 3.0x10^23 How many protons are there in 0.50 mol of O2? a.
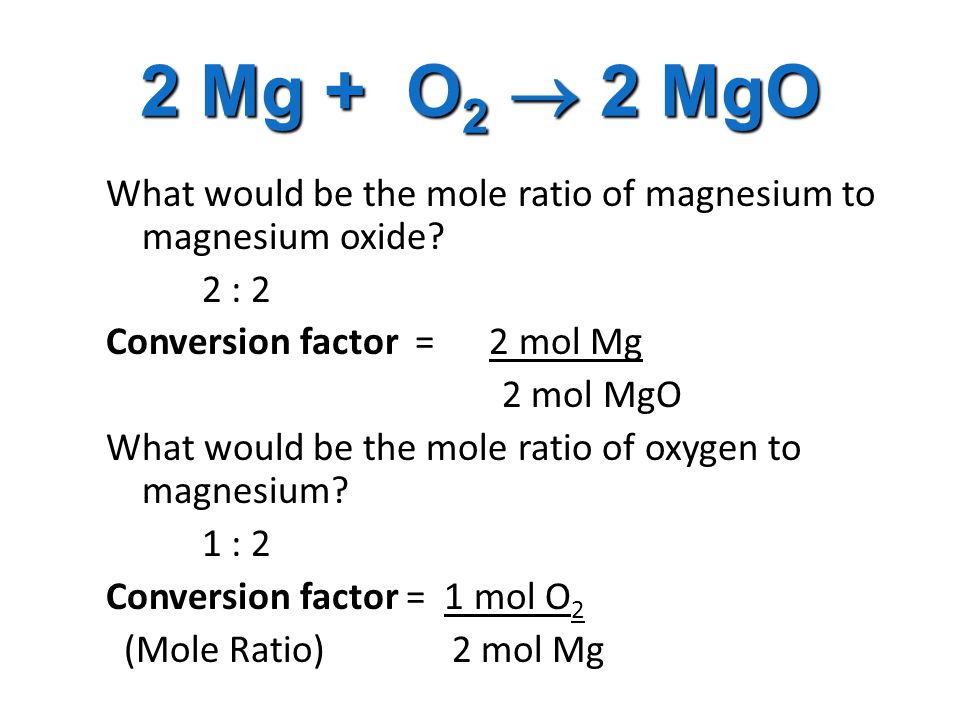
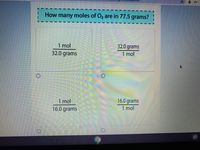
Mole Review 1.) Calculate the number of moles in 60.4L of O2. 2.) How many moles are there in 63.2g of Cl2? 1 mol O2 60.4L O2 = 2.7 mol O2 22.4L

SOLVED: How many grams of O2 are needed to produce 45.8 grams of Fe2O3 in the following reaction? The molar mass of O2 is 32.00 g/mole and the molar mass of Fe2O3