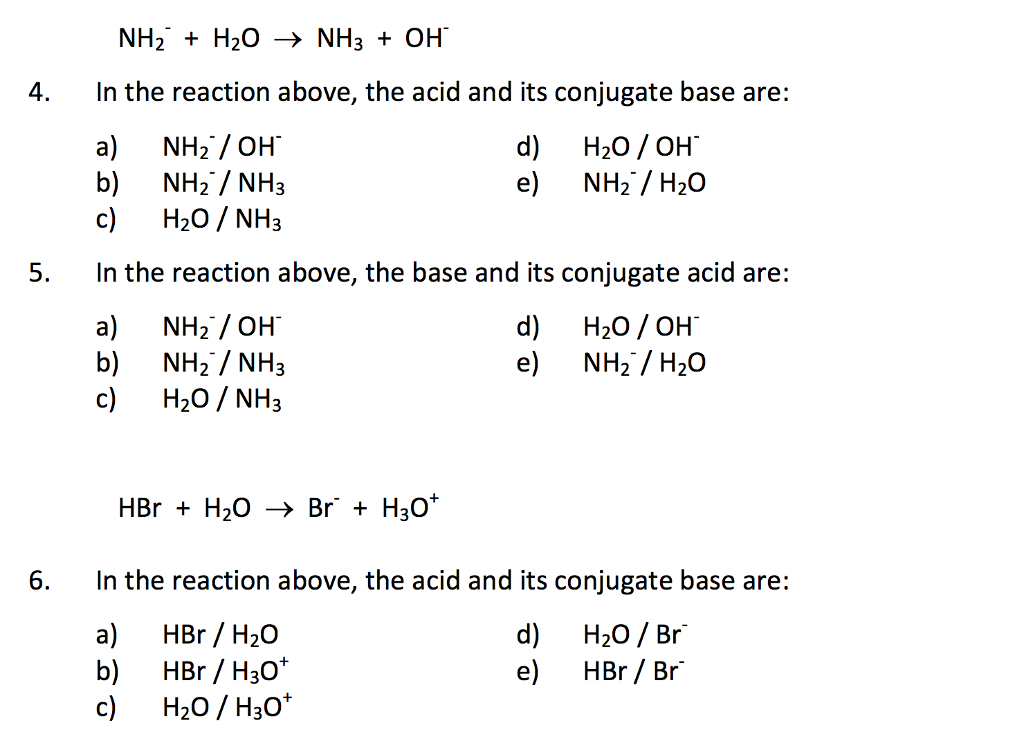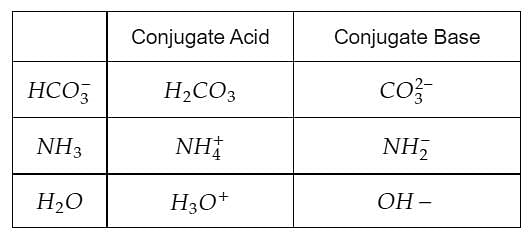
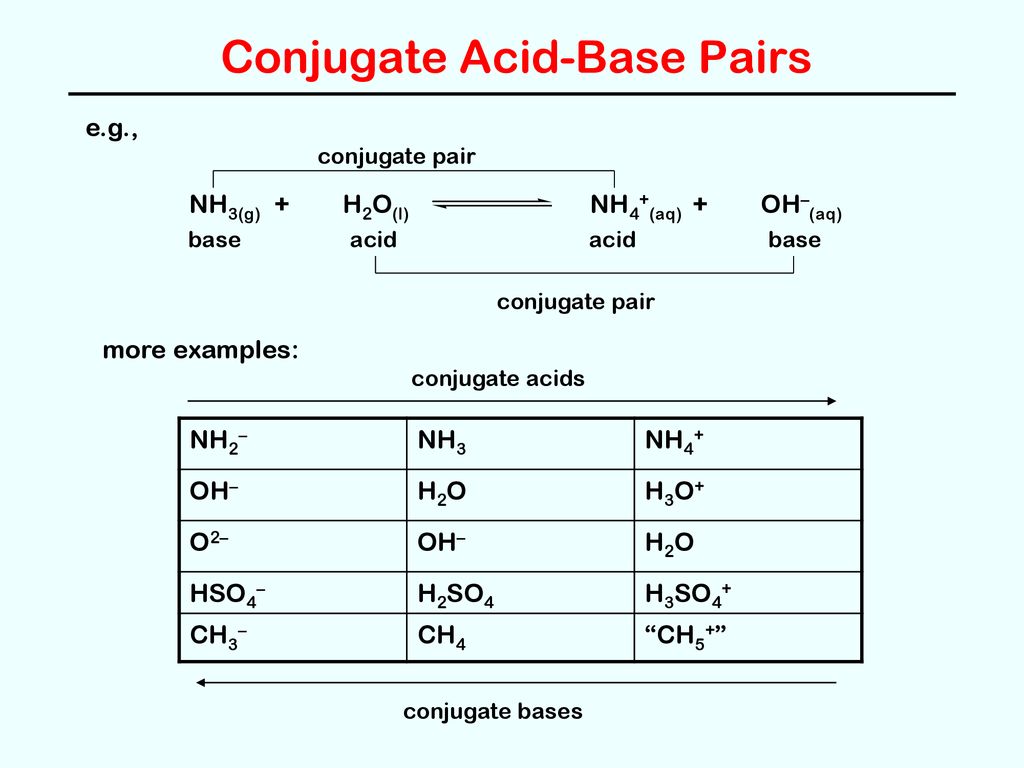
Which one of the following species can not act as both were bronsted acid and base? H2O NH2- NH3 HCO3-? | EduRev NEET Question

China Top Quality Fuse Base and Fuse Link Nh00 Nh1 Nh2 Nh3 Nh4 Photos & Pictures - Made-in-china.com

OneClass: Questions 3 and 4. Consider the reaction shown below: NH2-(ag) + H2O(l) ê·¼ NH3(gg) + OH-(a...

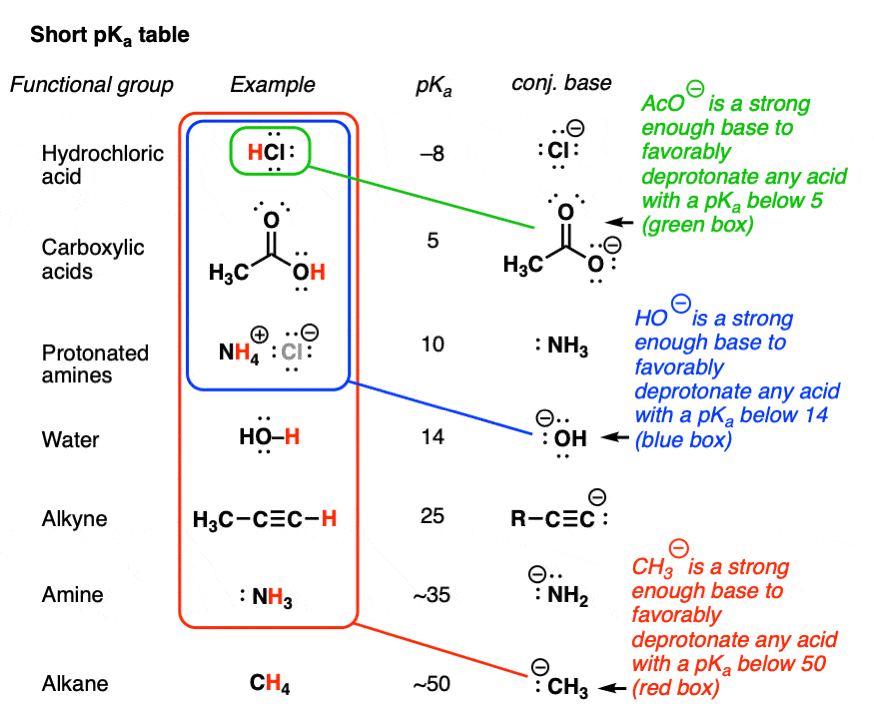
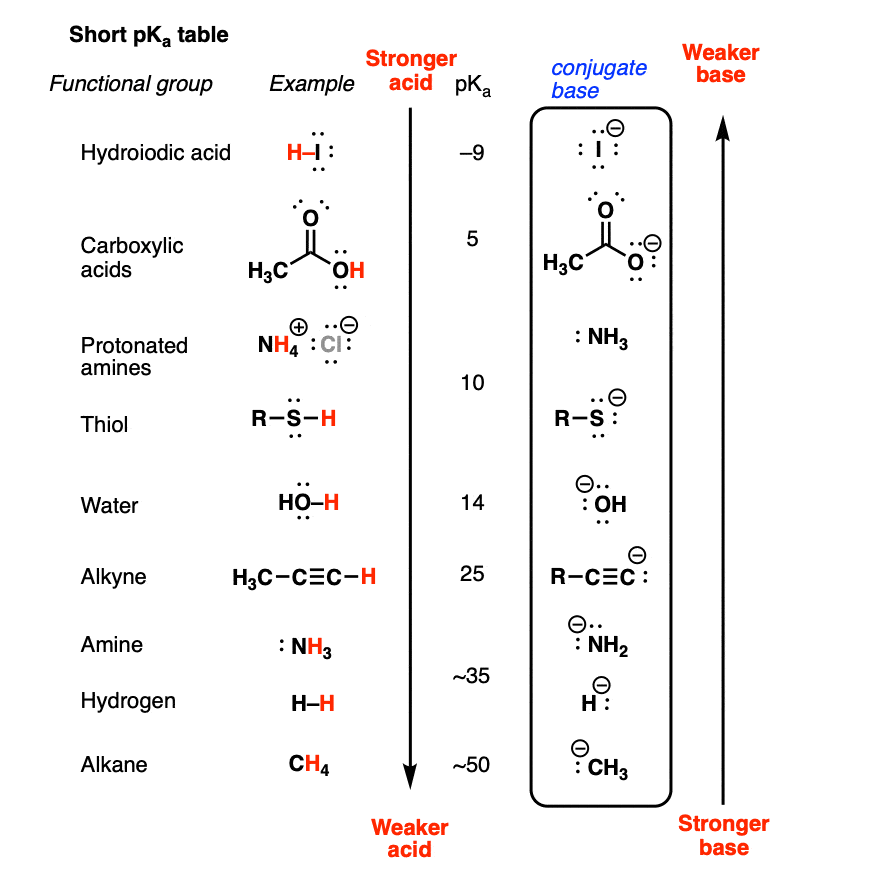
3.3: pKa of Organic Acids and Application of pKa to Predict Acid-Base Reaction Outcome - Chemistry LibreTexts

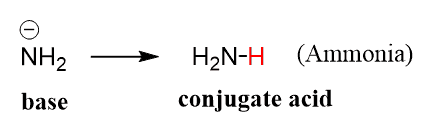
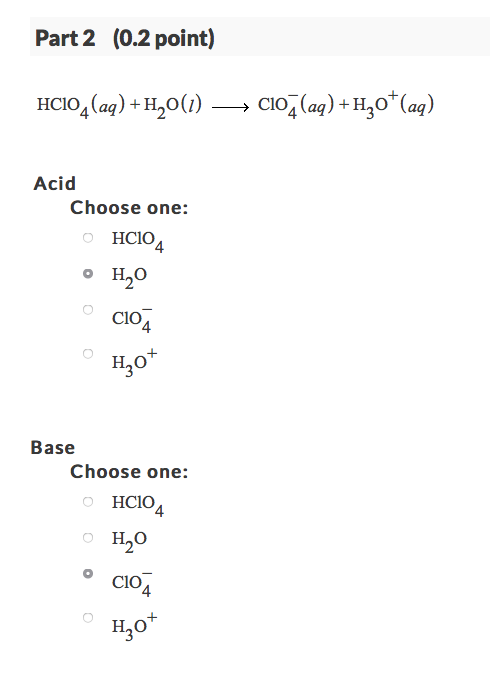



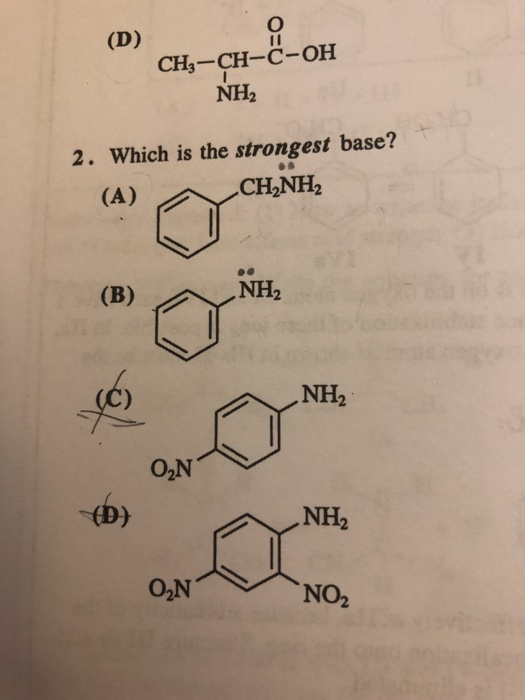
![Which is the correct for the following reaction: B (OH)3 + H2O → [B(OH)4]^ - + H^ + Which is the correct for the following reaction: B (OH)3 + H2O → [B(OH)4]^ - + H^ +](https://dwes9vv9u0550.cloudfront.net/images/5674386/ee9fa7d9-c7ef-4a7f-90dd-f3bb61402c59.jpg)