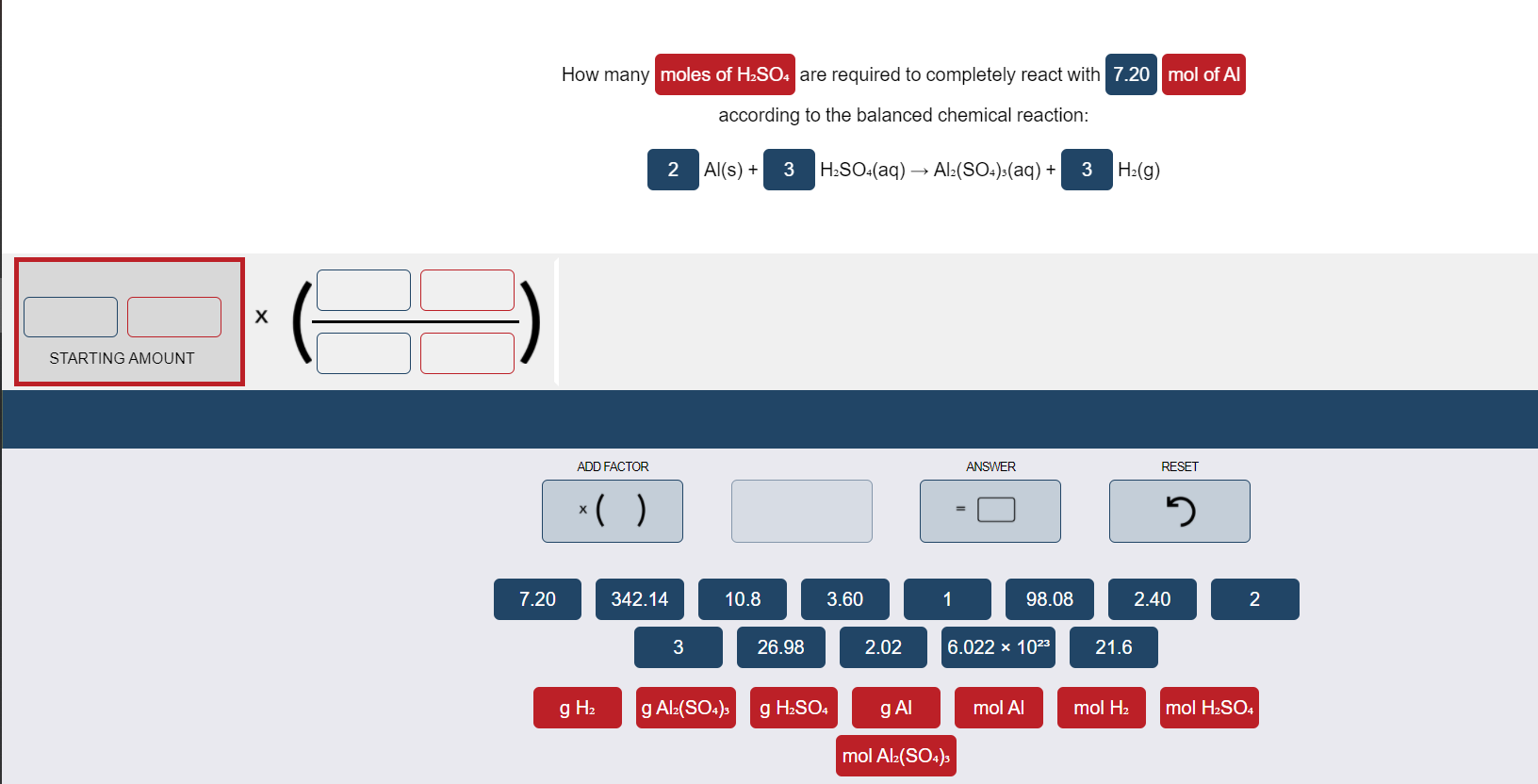
What volume of 0.250 mol/L sulfuric acid, H2SO4(aq) is needed to react completely with 37.2 mL of - Brainly.com
100 ml of 3 mol h2so4 reacts with 100 ml of 3 mol naoh. enthalpy of neutralisation of reaction will be 1) 57.1 kJ/ mol 2) 2 × 57.1 kJ 3) 0.3×57.1 kJ 4) 3×57.1 k

The molecular mass of H2SO4 is 98 amu. Calculate the number of moles of each elements in 294 g of H2SO4

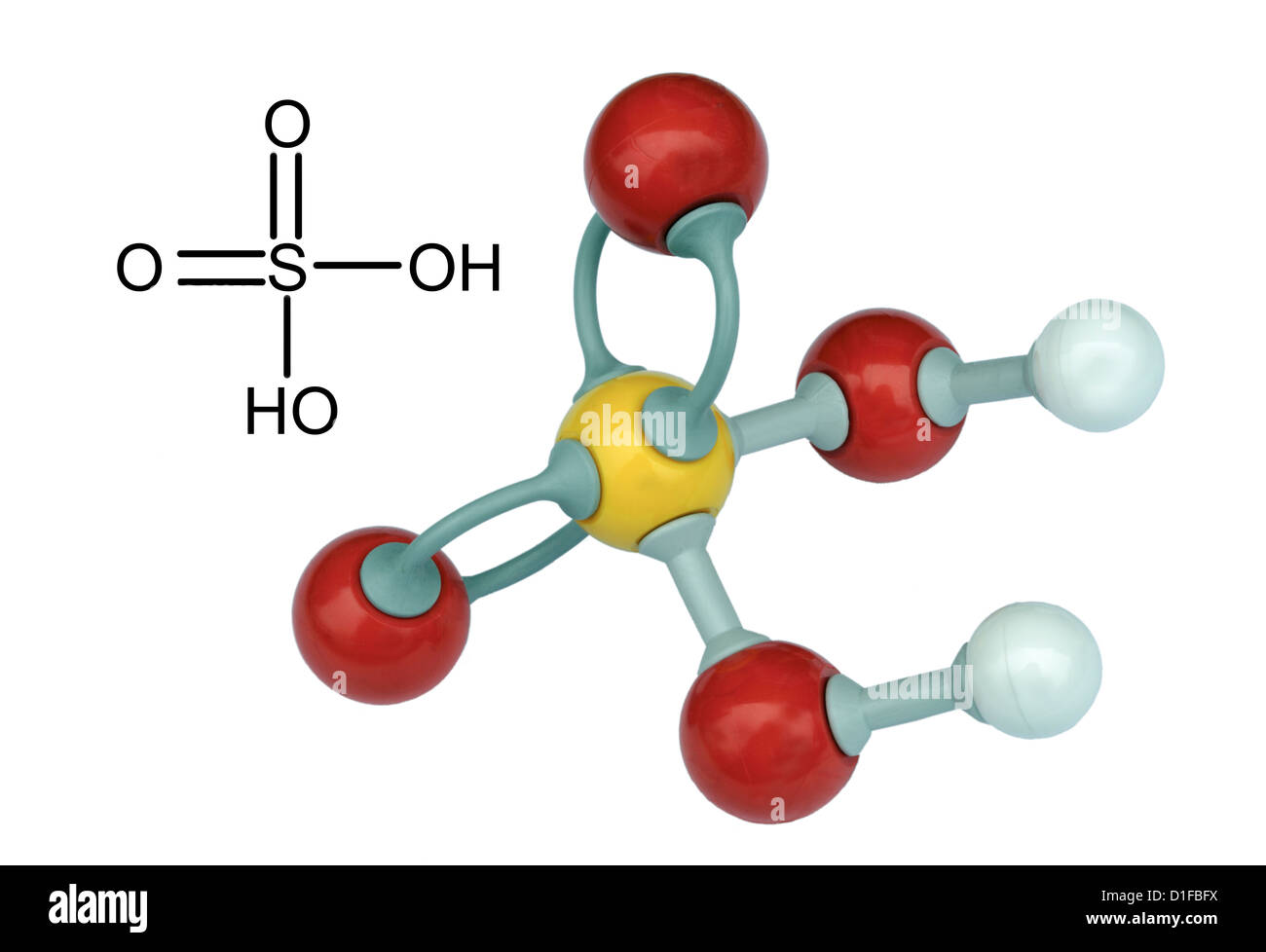
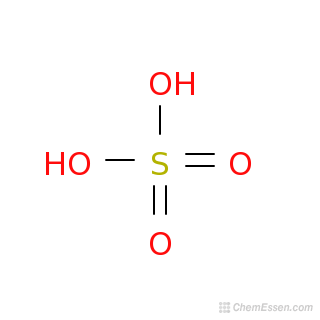
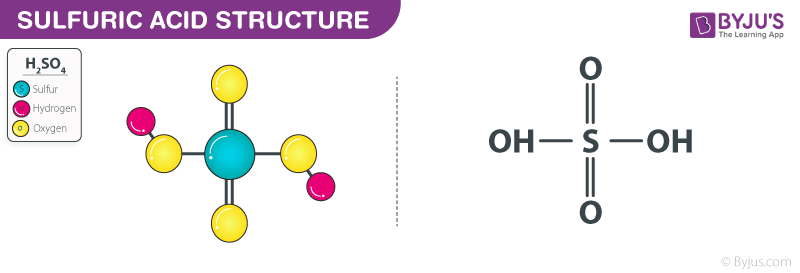
Sulfuric Acid, Sulphuric Acid, Is A Highly Corrosive Strong Mineral Acid With The Molecular Formula H2SO4, Vector 3d Molecular Structure Royalty Free SVG, Cliparts, Vectors, And Stock Illustration. Image 78444709.

how many moles of h2so4 are present in 4 9 g h2so4 - Chemistry - Some Basic Concepts of Chemistry - 12591081 | Meritnation.com



![Sulfuric Acid [H2SO4] Molecular Weight Calculation - Laboratory Notes Sulfuric Acid [H2SO4] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/11/sulfuric-acid-molecular-weight-calculation.jpg)