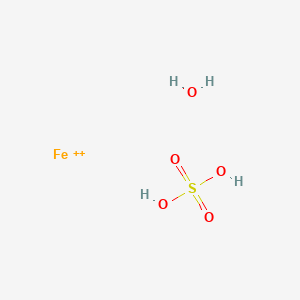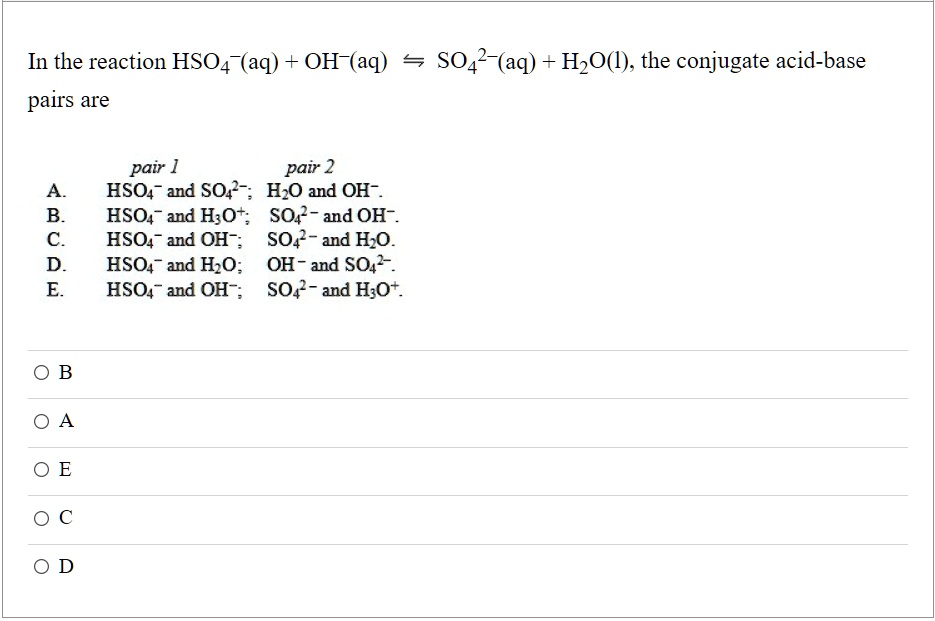
SOLVED: In the reaction HSO4 (aq) + OH-(aq) 5 SO42-(aq) H2O(l), the conjugate acid-base pairs are pair 1 pair 2 HSO- and SO4 -; H,O and OH- HSO4- and H;O;; SO4 -

Table 2 from H 2 O ) · H 2 O : Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Semantic Scholar
Al + KMnO4 + H2SO4 = KHSO4 + Al2(SO4)3 + MnSO4 + H2O KNO3 + FeSO4 + H2SO4 = KHSO4 + Fe2(SO4)3 + NO H2O H2S + K2Cr2O7 + H2SO4 =KHSO4 + Cr2(SO4)3 + S + H2O Balance the equations using ion electron method.
![SOLVED: [Cu(NH3)4]SO4 • H20 (s) was prepared from 15.5 grams CuSO4 • 5 H2O (s) using a total of 10 mL of 15 M NH3. Determine which reactant is limiting. Molar Masses: SOLVED: [Cu(NH3)4]SO4 • H20 (s) was prepared from 15.5 grams CuSO4 • 5 H2O (s) using a total of 10 mL of 15 M NH3. Determine which reactant is limiting. Molar Masses:](https://cdn.numerade.com/ask_previews/3e5daa89-75dc-40aa-92e7-425d1e093c86_large.jpg)
SOLVED: [Cu(NH3)4]SO4 • H20 (s) was prepared from 15.5 grams CuSO4 • 5 H2O (s) using a total of 10 mL of 15 M NH3. Determine which reactant is limiting. Molar Masses:

Solubility of FeSO4·7H2O in the H2SO4–Ti(SO4)2–H2O, H2SO4–MgSO4–H2O, and HCl–H2O Systems from 278 to 313 K | Journal of Chemical & Engineering Data

Fe(OH)3 + H2SO4(dil.) = Fe2(SO4)3 + H2O Is it a balanved equation? what are the number of atoms of the - Brainly.in
![Give the oxidation state, d - orbital occupation and coordination number of the central metal ion in the following complex. [Mn(H2O)6]SO4 . Give the oxidation state, d - orbital occupation and coordination number of the central metal ion in the following complex. [Mn(H2O)6]SO4 .](https://haygot.s3.amazonaws.com/questions/1873456_1575827_ans_3125df065a114c8e8610e9410b778e2a.jpeg)




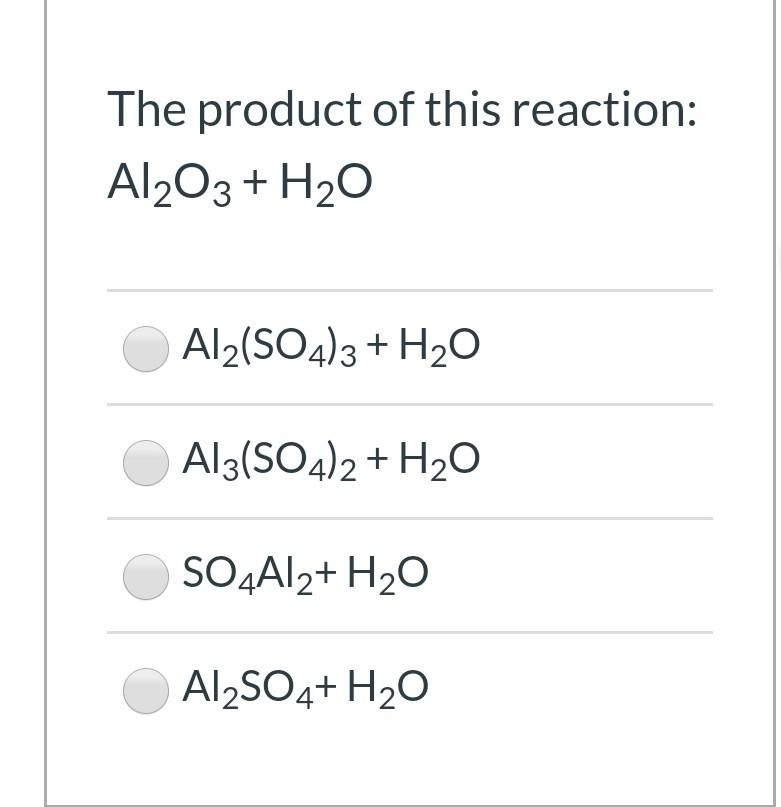
![Answered: a. CГОЗ HO H,SO4, H2O b. [1] O3 [2] H20… | bartleby Answered: a. CГОЗ HO H,SO4, H2O b. [1] O3 [2] H20… | bartleby](https://content.bartleby.com/qna-images/question/d66f1a49-6a74-4120-82f6-39c04434f2ae/0caae1aa-a18d-4475-b945-b3b8c68a132a/nw3bhka.png)