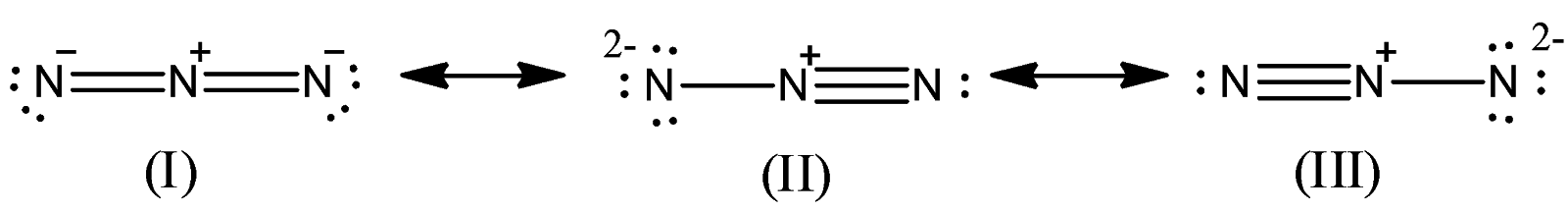
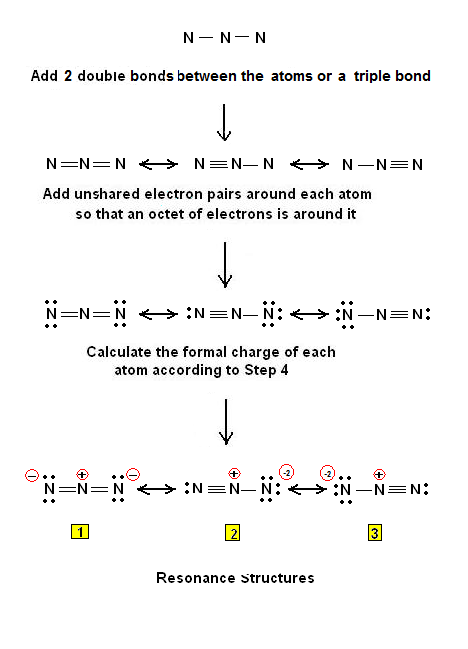
Azide ion (${{N}_{3}}^{-}$) exhibits an N-N bond order of 2 and may be represented by resonance structures I, II and III given below. Select correct statements.\n \n \n \n \n (A) Structures

The azide ion, n−3, is a symmetrical ion, all of whose contributing structures have formal charges. - Brainly.com

Draw and explain the Lewis structure for the azide ion, showing all its possible resonance structures. Give the name of the molecular geometry and the hybridization around the central atom. | Homework.Study.com
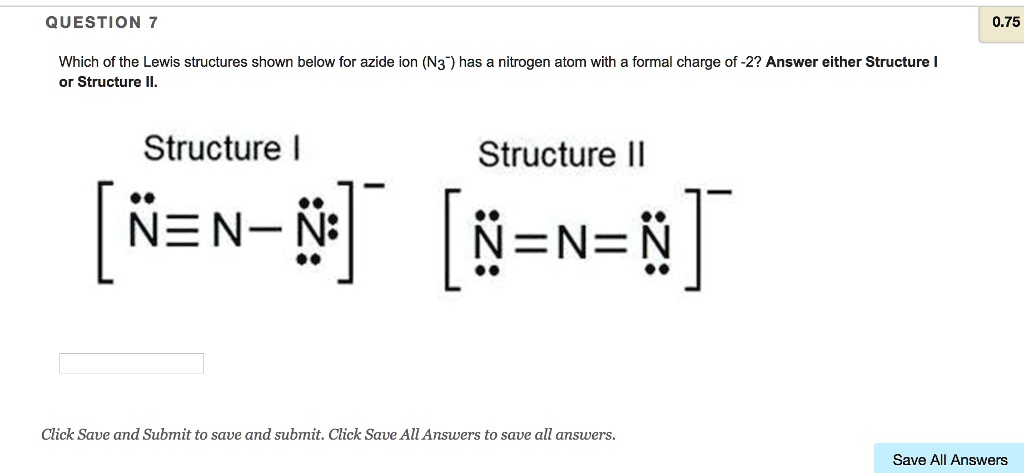
SOLVED: QUESTION 7 0.75 Which of the Lewis structures shown below for azide ion (N3 has nitrogen atom with formal charge of -2? Answer either Structure or Structure II: Structure NEN-N Structure

Draw and explain the Lewis structure for the azide ion, showing all its possible resonance structures. Give the name of the molecular geometry and the hybridization around the central atom. | Homework.Study.com





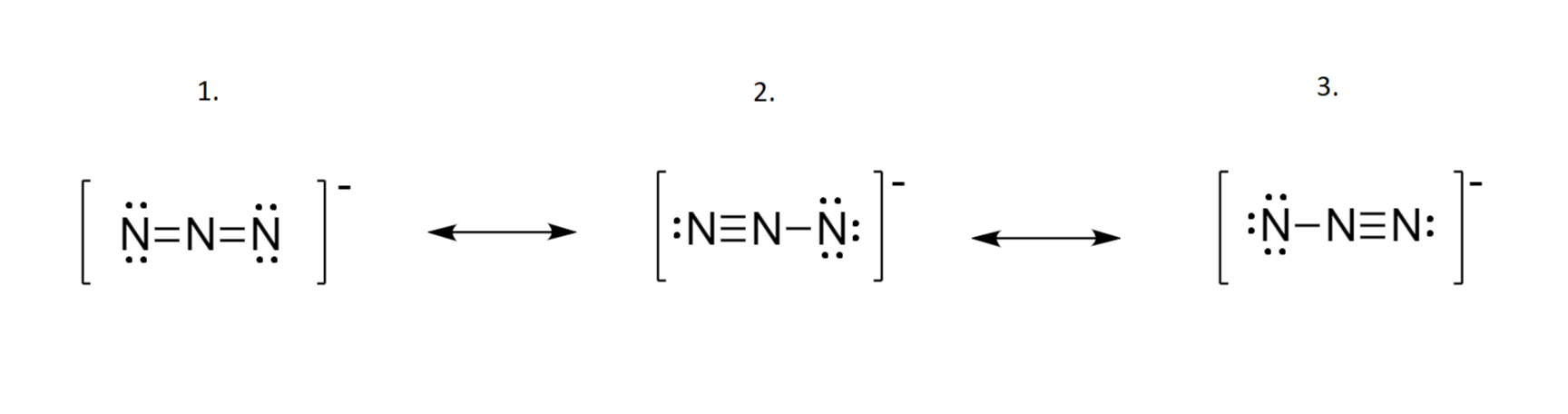
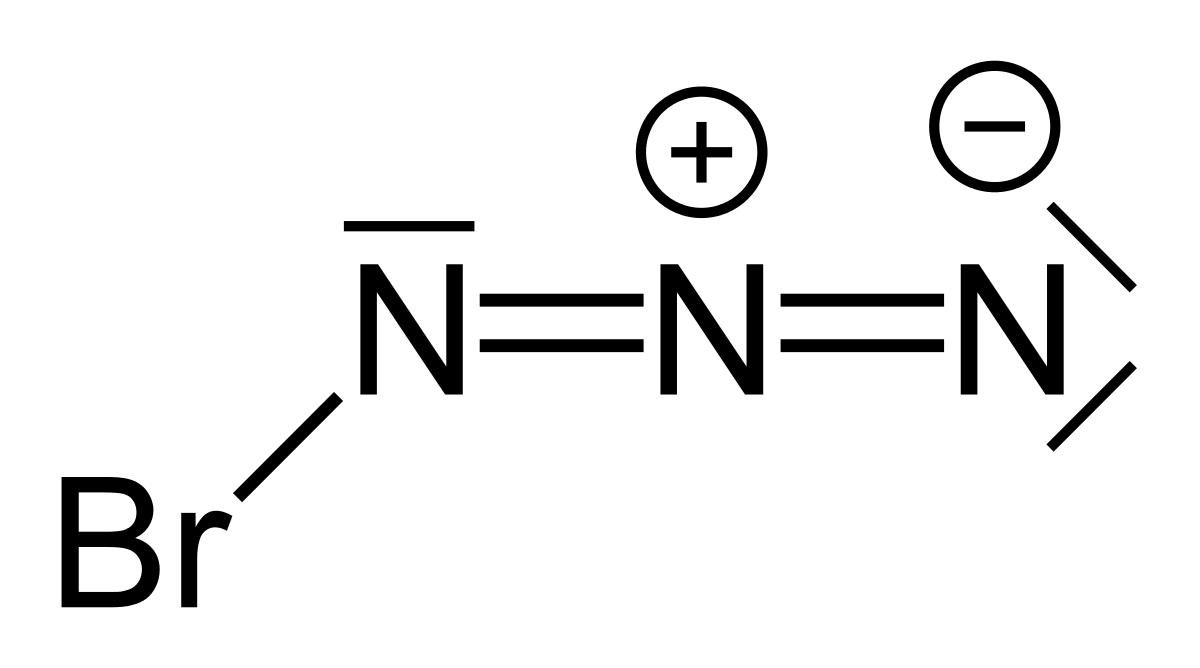
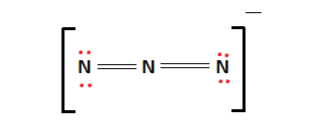

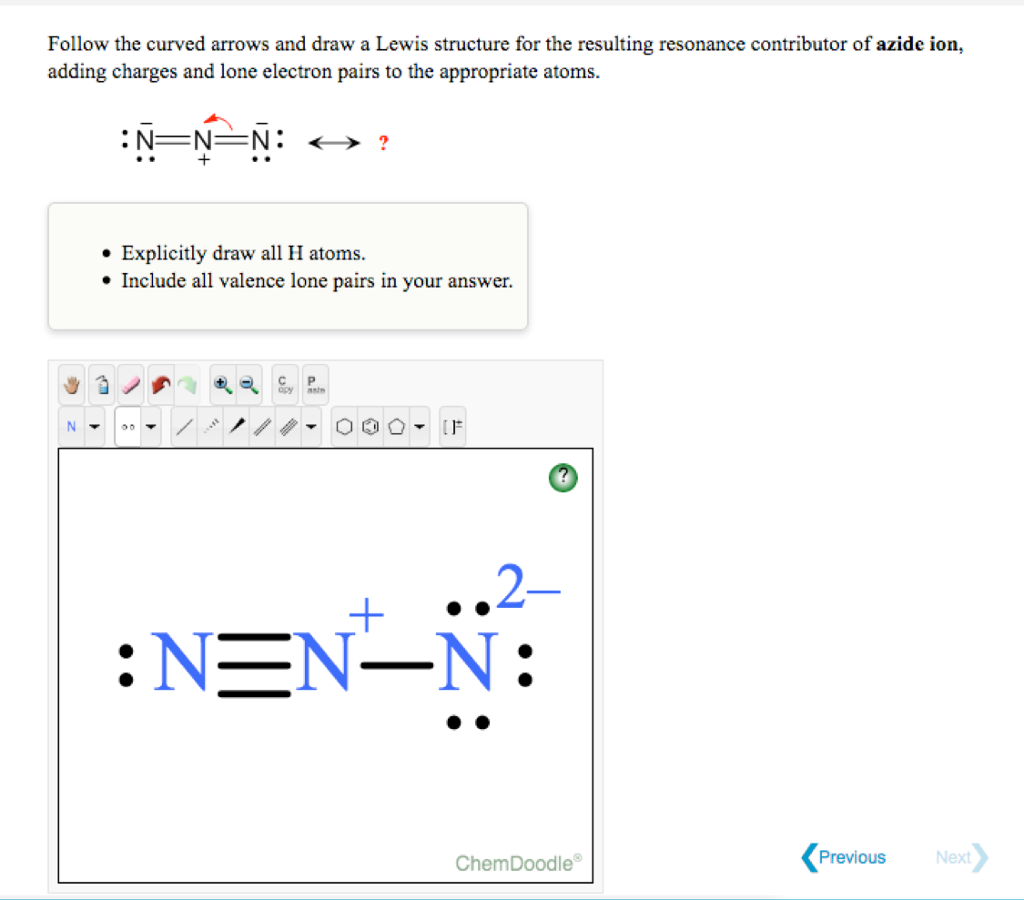


![Azides [N3(–)] - ChemistryScore Azides [N3(–)] - ChemistryScore](https://chemistryscore.com/wp-content/uploads/2019/11/Azides-N33-1024x206.png)