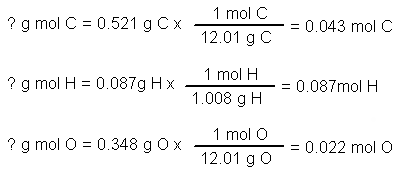
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are
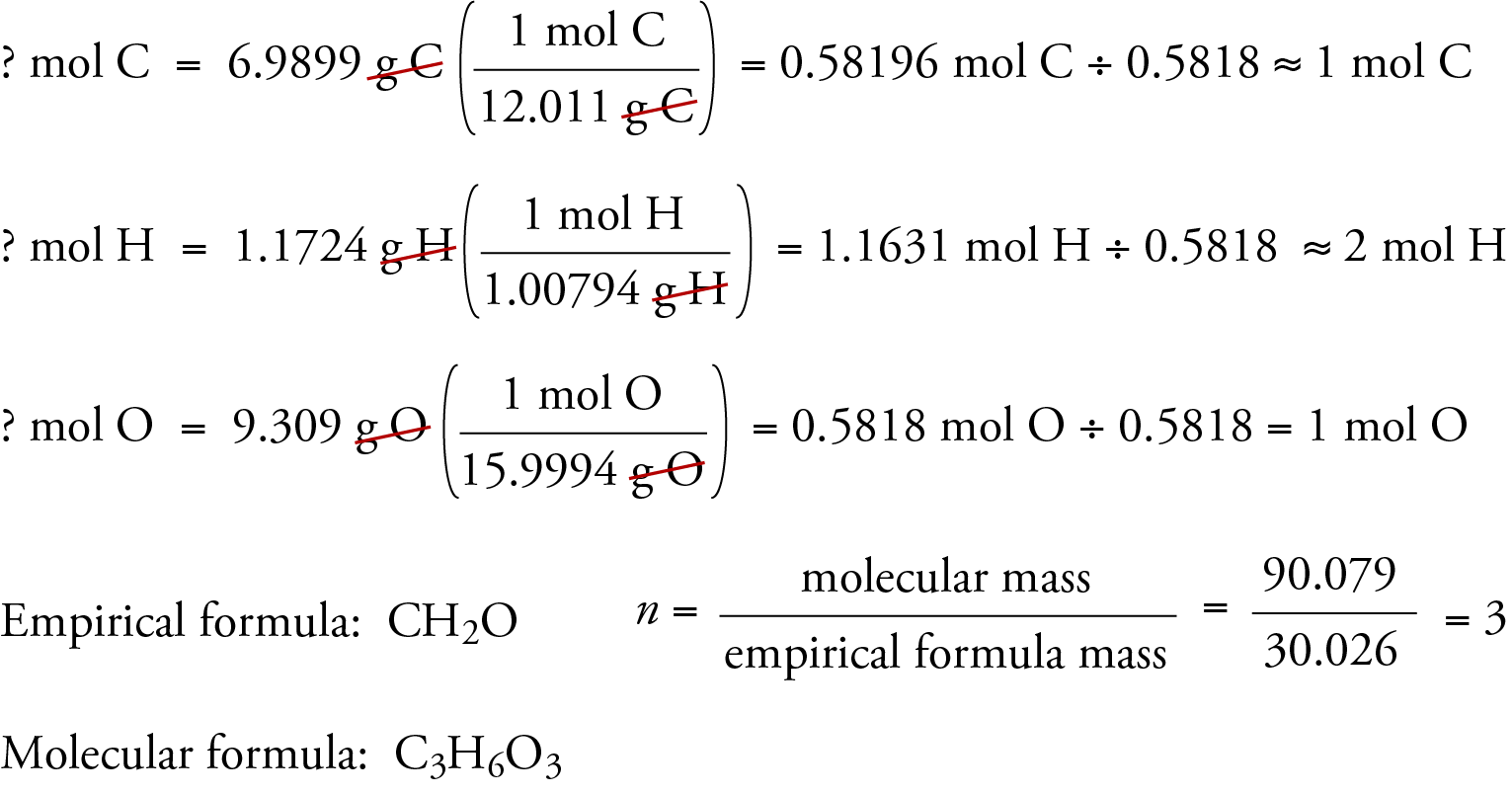
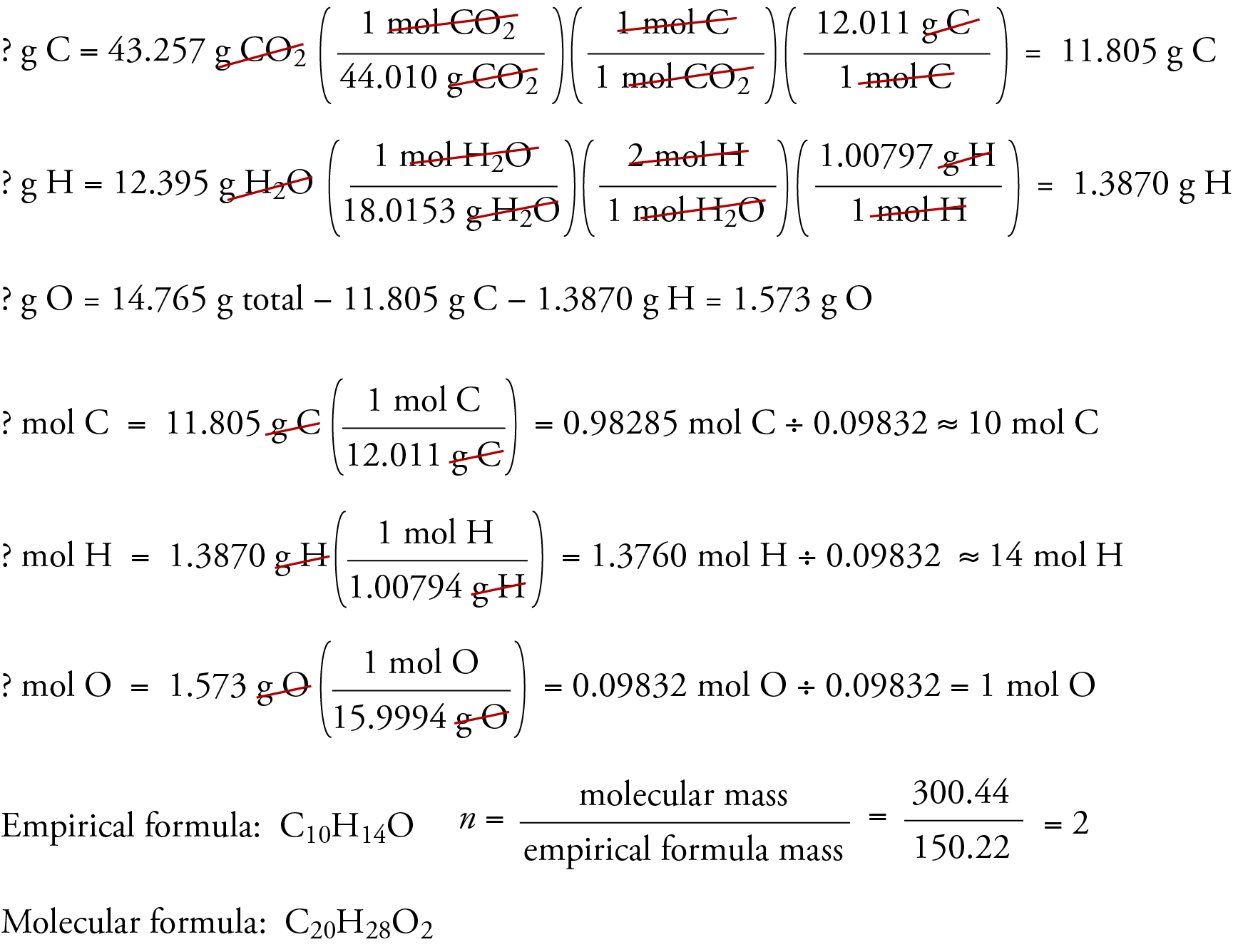
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are

SOLVED: Determine the number of moles of oxygen atoms in each sample. a. 4.88 mol H2O2 b. 2.15 mol N2O c. 0.0237 mol H2CO3 d. 24.1 mol CO2

SOLVED: How many of each the following contained in 100 grams of CO2 (m=44.01)? calculate mol of C,O and O2
Objective: Define empirical formula, and explain how the term applies to ionic and molecular compounds

Bisphenol A, molecular formula: C 15 H 16 O 2, molar mass is 228.29 g/mol. | Download Scientific Diagram








:max_bytes(150000):strip_icc()/pancit-molo-recipe-5209963-hero-01-c89a8ced0df74edb99b4379d413fca58.jpg)