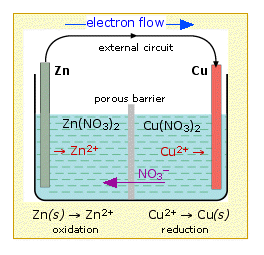
THE GROUND STATE Cu2+ION AFFINITIES OF GLYCINE, ALANINE AND CYSTEINE IN GAS AND AQUEOUS PHASE: A DFT BASED COMPUTATIONAL STUDY

Why is Cu+ diamagnetic while Cu2+ is paramagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
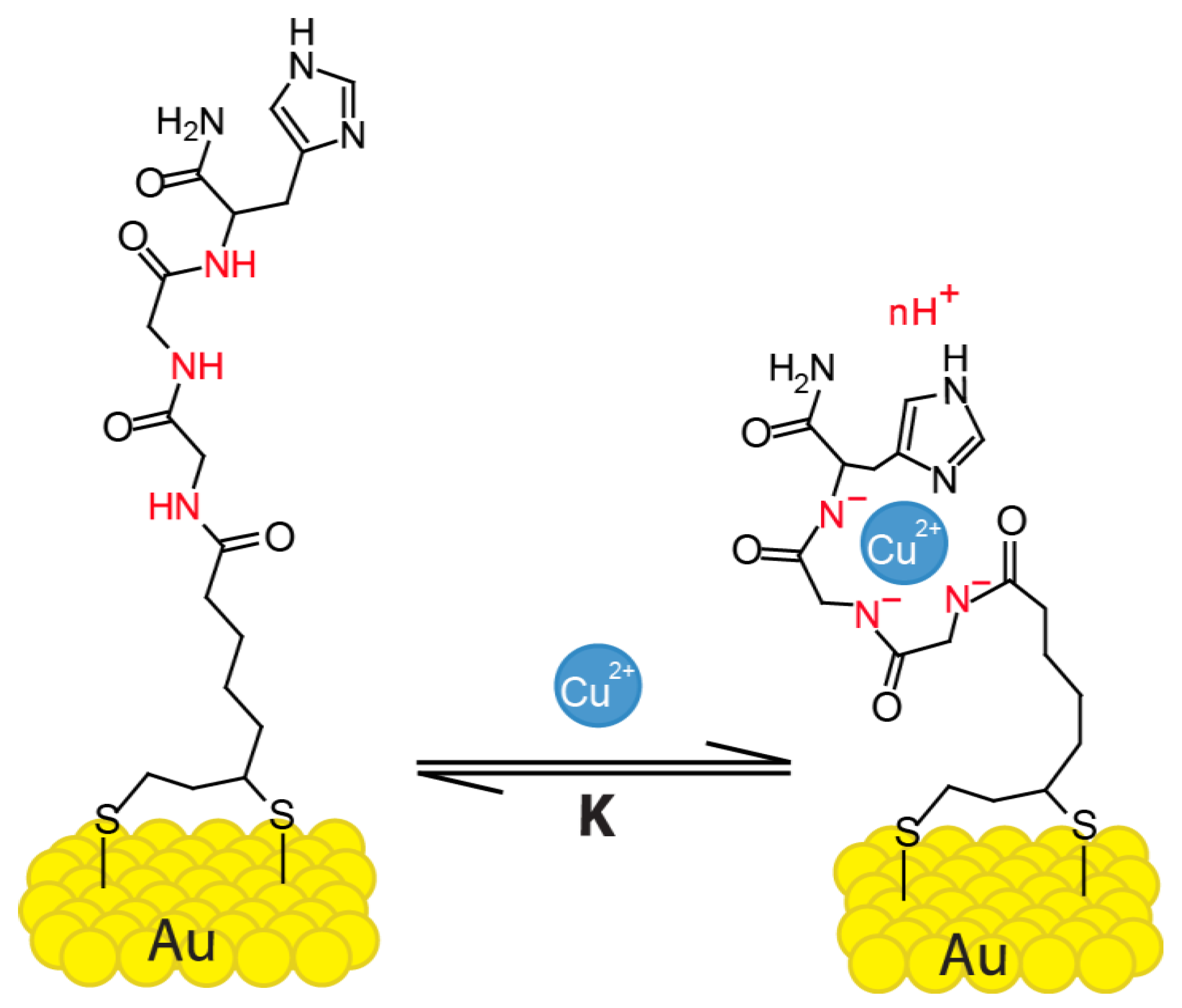
Sensors | Free Full-Text | Detection of Cu2+ Ions with GGH Peptide Realized with Si-Nanoribbon ISFET

Give the correct formula for the compound formed by the combination of the Cu2+ and SO42- ions. | Homework.Study.com

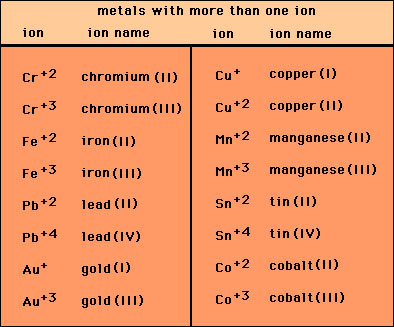
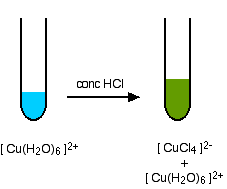


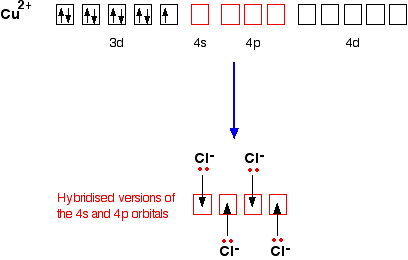



![For a complex ion, [Cu(NH3)4]^2 + : For a complex ion, [Cu(NH3)4]^2 + :](https://haygot.s3.amazonaws.com/questions/1731528_b11046ad9936443990f5efe5a262d1e8.png)