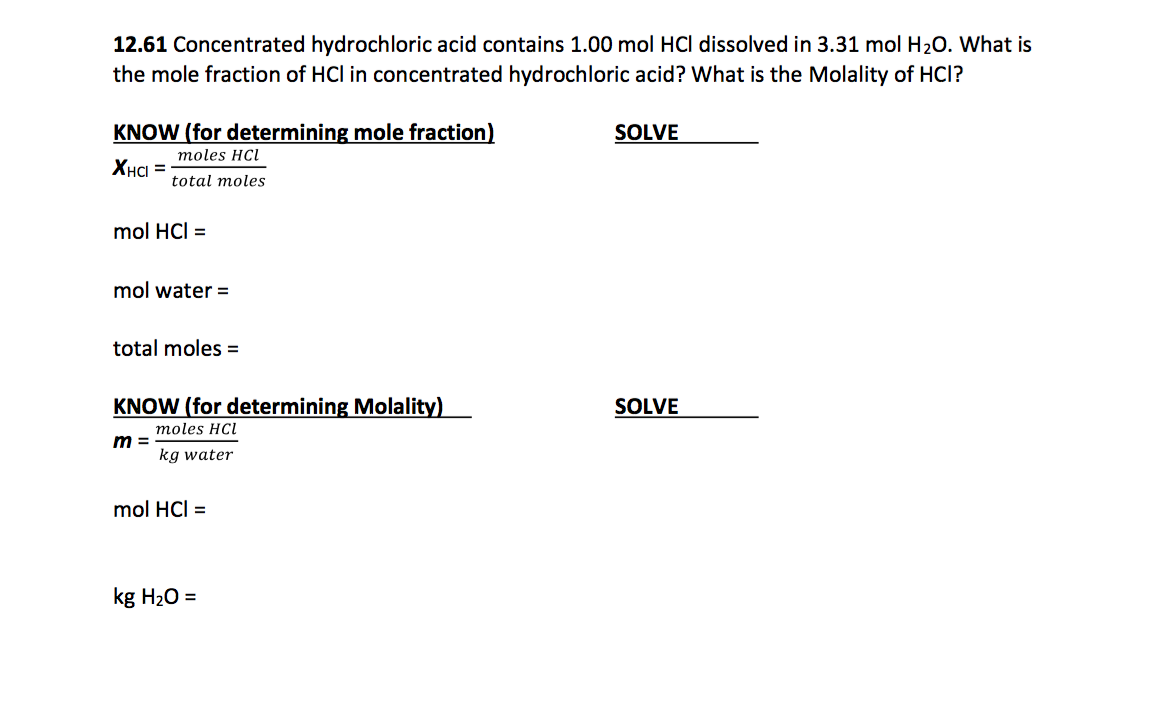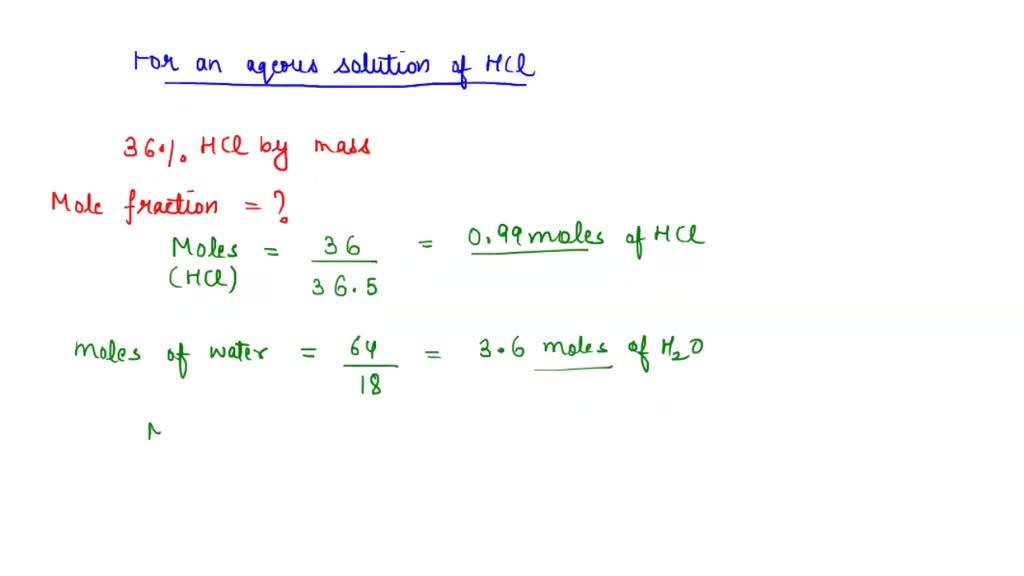
SOLVED: Sample Exercise 13.6 Calculation of Mole Fraction and Molality An aqueous solution of hydrochloric acid contains 36% HCl by mass. (a) Calculate the mole fraction of HCl in the solution. (b)

If the enthalpy of formation and enthalpy of solution of HCl (g) are-92.3kj /mol and -75.14kJ/mol - YouTube
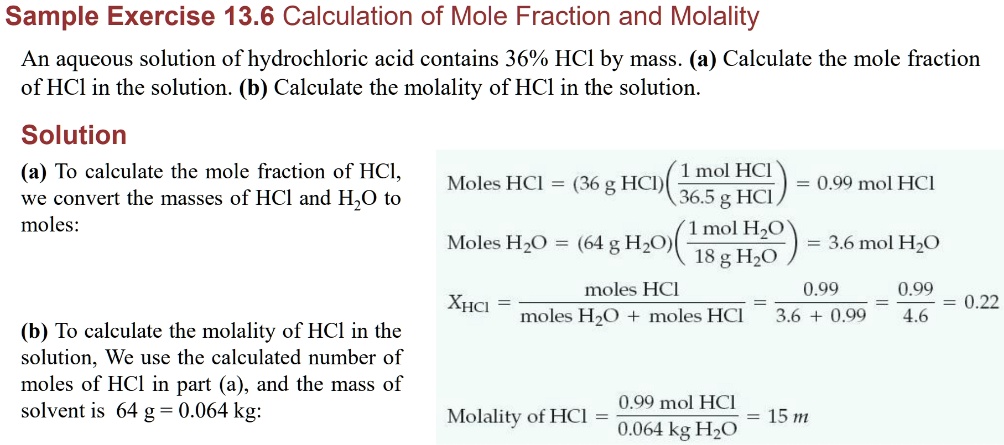
SOLVED: Sample Exercise 13.6 Calculation of Mole Fraction and Molality An aqueous solution of hydrochloric acid contains 36% HCl by mass. (a) Calculate the mole fraction of HCl in the solution. (b)

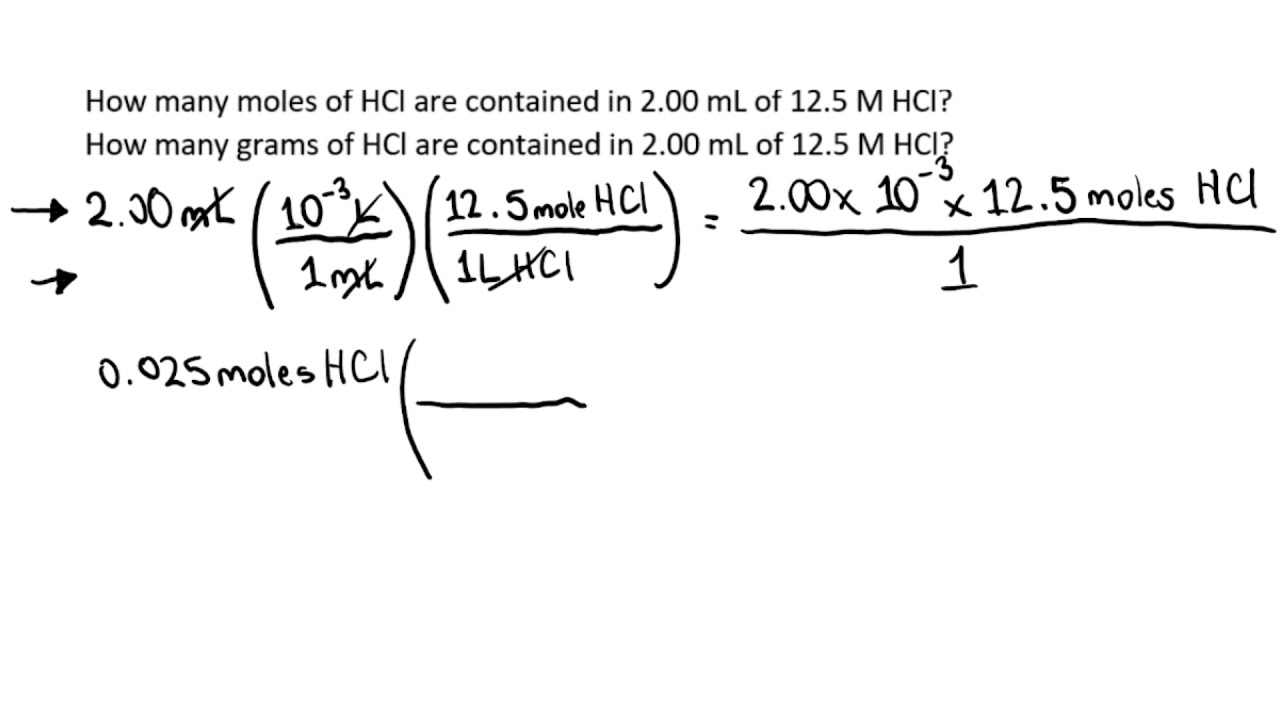
One liter of 5.0 M HCl contains how many moles of HCl? M = 5.0 mol= ? L = 1 L x = 5 mol. - ppt download
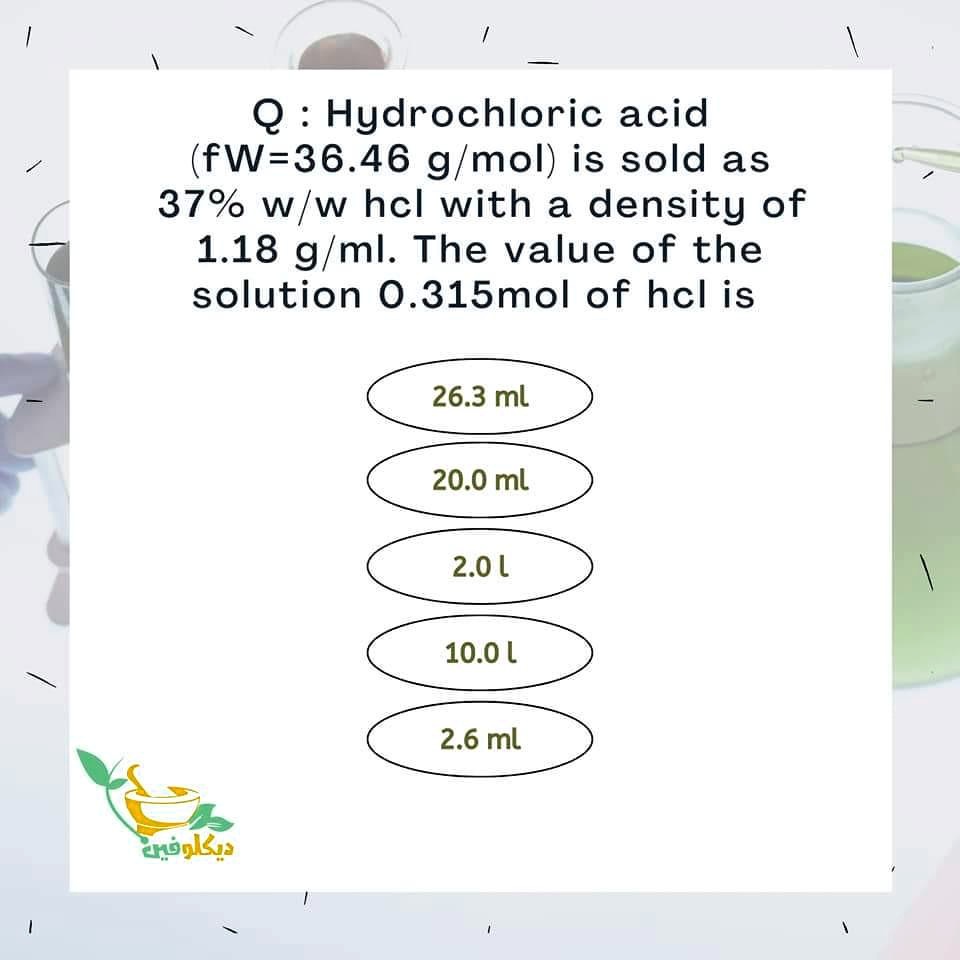
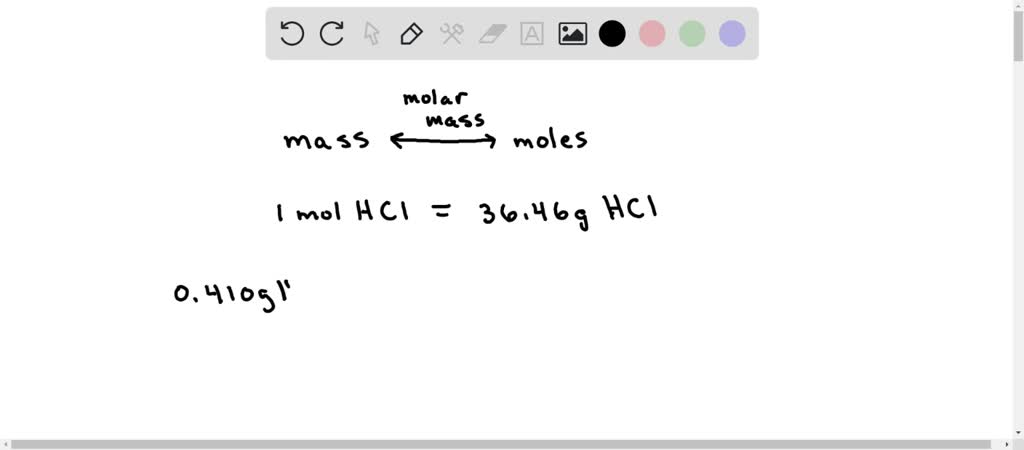
SOLVED: Q : Hydrochloric acid (FW=36.46 g/mol) is sold as 37% ww hcl with a density of 1.18 g/ml: The value of the solution 0.315mol of hcl is 26.3 ml 20.0 ml 2.0 ( 10.0 ( 2.6 ml ehogkky?

How many gram moles of HCl will be required to prepare one litre of a buffer solution (containing NaCN and HCN ) of pH 8.5 using 0.10g formula mass of NaCN ?


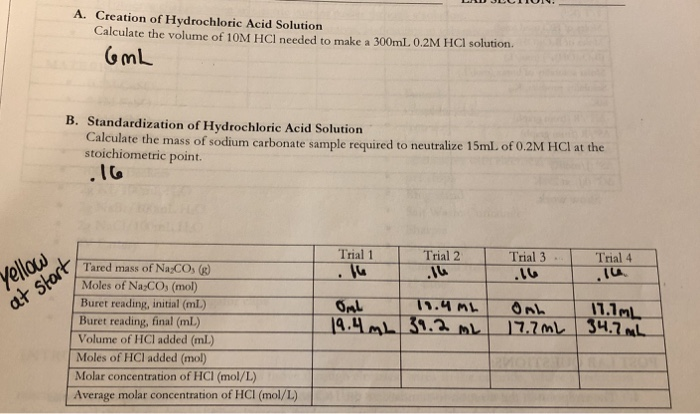


![Solution enthalpy of (CaHap) in the hydrochloric acid solution [HCl]/... | Download Table Solution enthalpy of (CaHap) in the hydrochloric acid solution [HCl]/... | Download Table](https://www.researchgate.net/publication/243957100/figure/tbl1/AS:668975847452674@1536507679338/Solution-enthalpy-of-CaHap-in-the-hydrochloric-acid-solution-HCl-mol-L-1.png)