125 mL of 63% (w/v) H2C2O4 2H2O solution is made to react with 125 mL of a 40%(w/v) NaOH solution - Chemistry - Redox Reactions - 11941859 | Meritnation.com
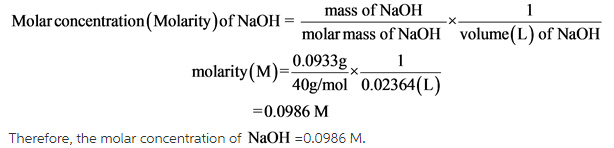
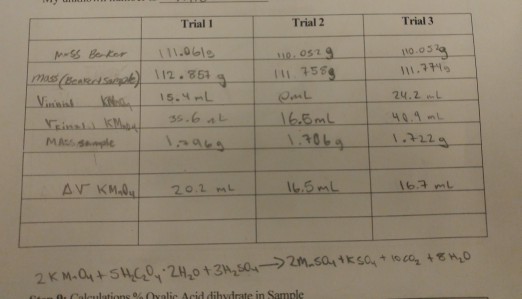
Oxalic acid, H2C2O4*2H2O (molar mass = 126.07 g/mol) is oftenused as a primary standard - Home Work Help - Learn CBSE Forum
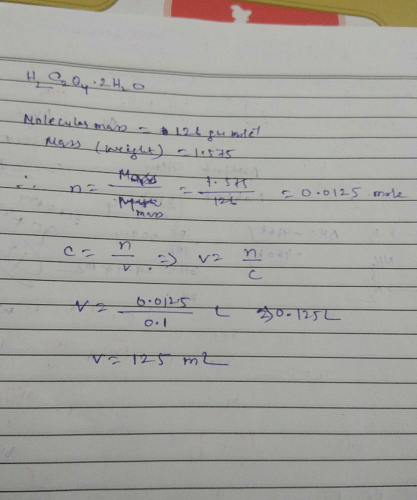
Volume (mL) of H2C2O4. 2H2O solution to prepare 0.10 M from 1.575g of it is ?Correct answer is '125'. Can you explain this answer? | EduRev Chemistry Question

Oxalic acid, H2C2O4*2H2O molar mass = 12607 g/mol is often used as a primary standard for the sta - YouTube
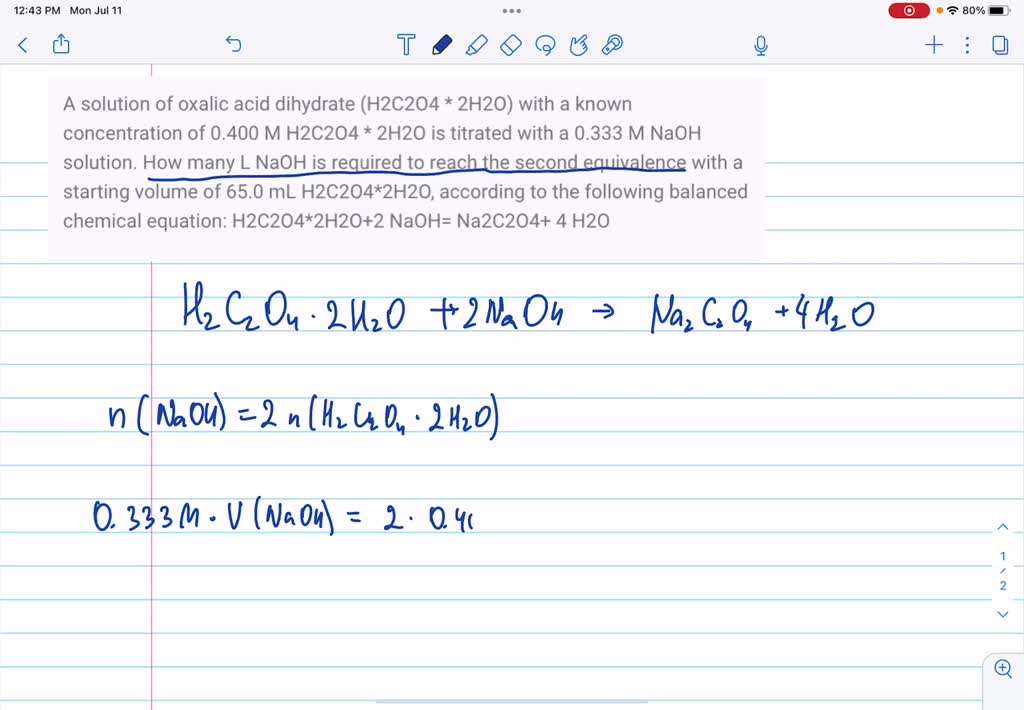
SOLVED: How much oxalic acid (H2C2O4 with 2H2O) is required to prepare 1000ml of solutions of the following respective normalities: a.)0.5000, b.)0.2500, c.)0.200?
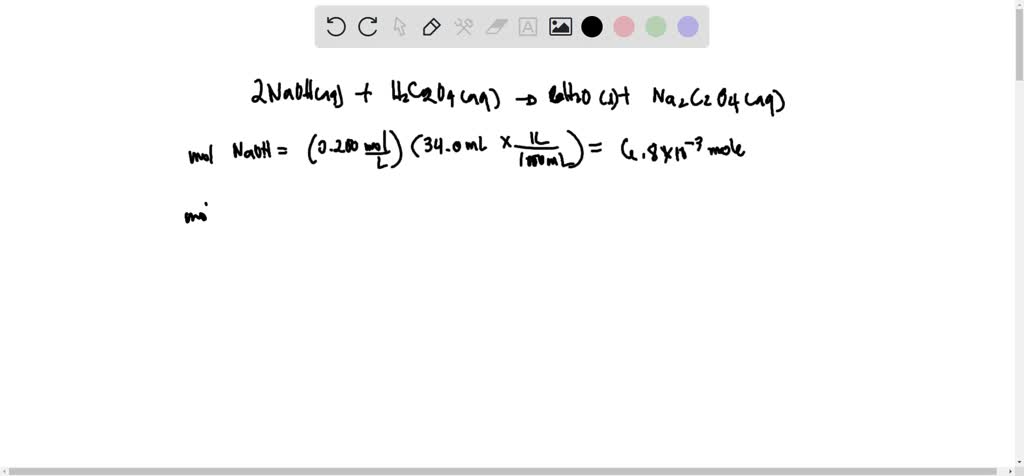

![Oxalic Acid Dihydrate [H2C2O4 2H2O ] [CAS_6153-56-6] 99.6+% Fine White – Wintersun Oxalic Acid Dihydrate [H2C2O4 2H2O ] [CAS_6153-56-6] 99.6+% Fine White – Wintersun](https://cdn.shopify.com/s/files/1/0724/7981/products/15-005-4_1024x1024.jpg?v=1662152063)