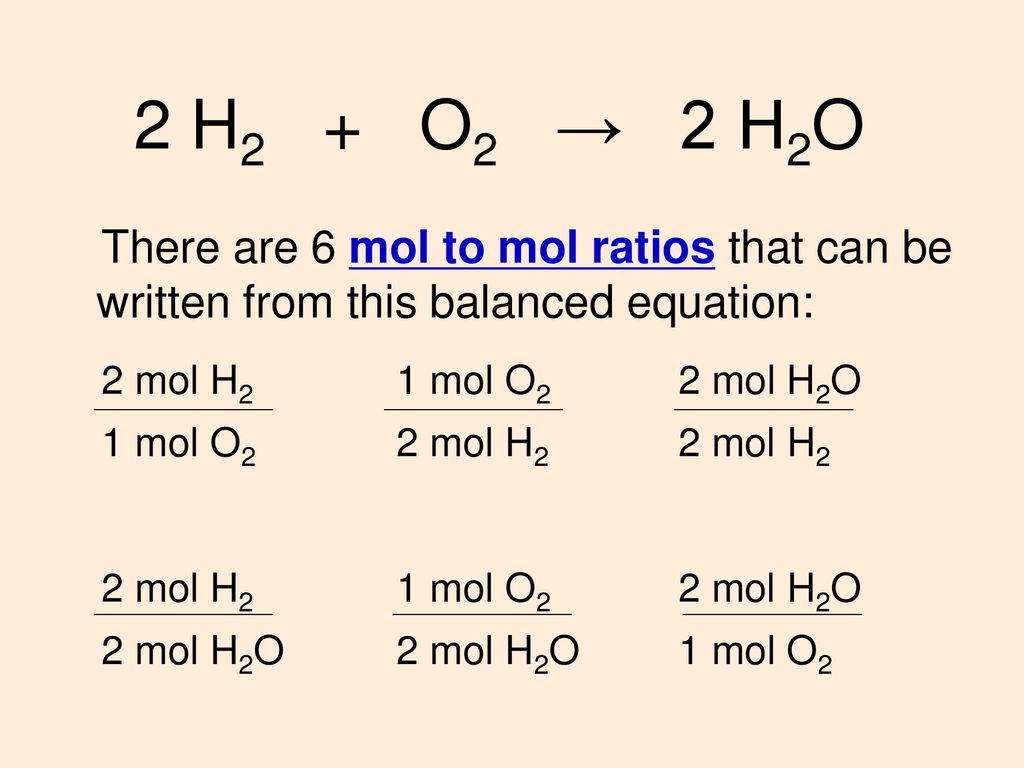
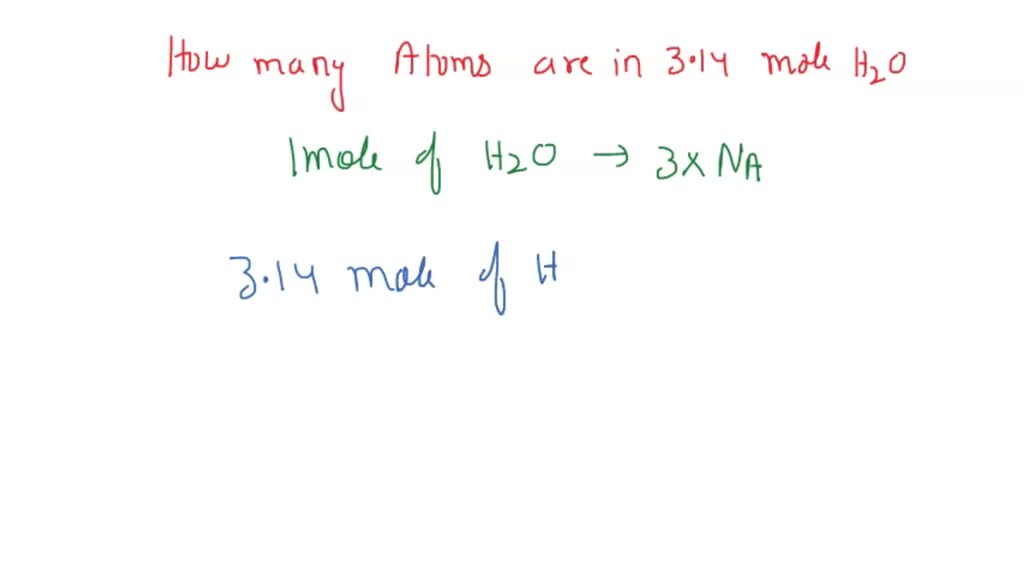In the reaction 2 H2 + O2m 2 H20, howmany moles of oxygen are required to fullyreact with 6.0 mol of - Brainly.com
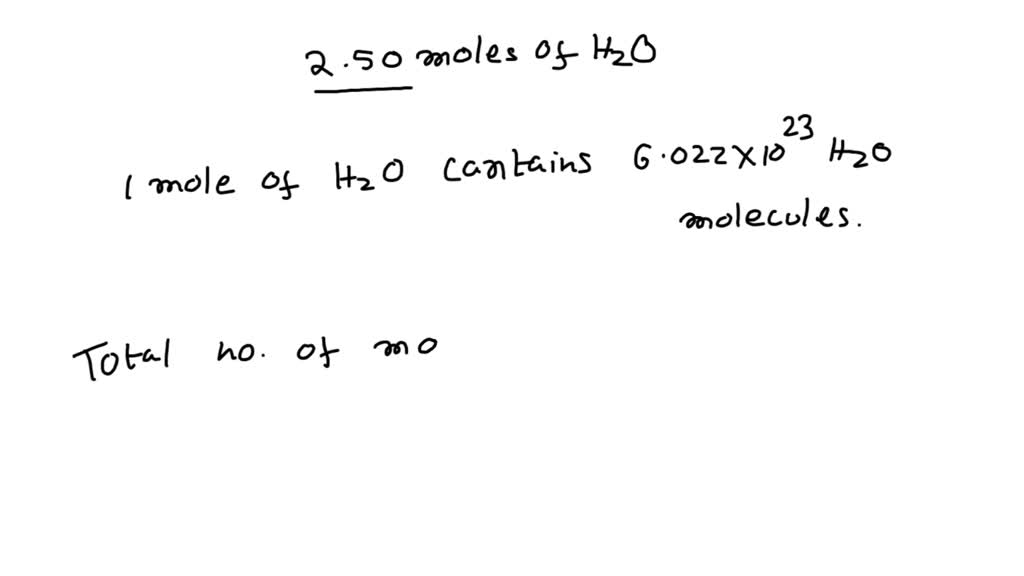
SOLVED: How many molecules are in 2.50 moles of H2O? 0 1.51 X 1024 molecules of H20 0 4.15 X 10-24 molecules of H20 0 3.01 X 1024 molecules of H20 O 8.31 X 10-24 molecules of H20

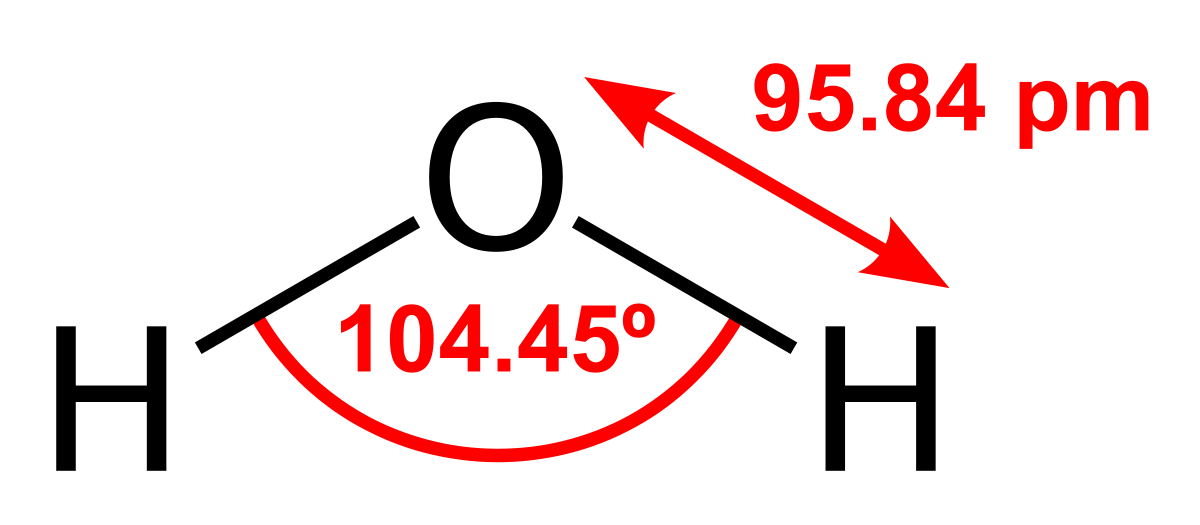
The enthalpy change on freezing of 1 mol of water at 5^oC to ice at - 5^oC is : ( Given ΔfusH = 6 kJ mol^-1 at 0^oC , Cp(H2O, l) =

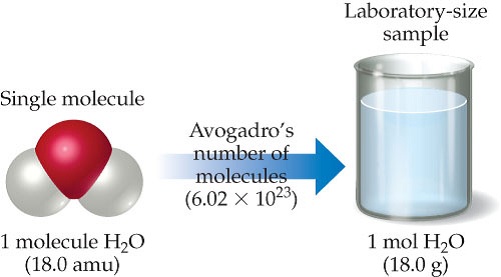
The molar mass of H2O is 18.01 g/mol . The molar mass of 02 is 32.00 g/mol What is the mass of H20 in - Brainly.com

SOLVED: How many moles of hydrogen atoms are present in a sample that contains 3.64 moles of water, H2O? answer=mol H How many moles of H2O molecules are present in a sample

SOLVED: 1) How many molecules of H2O are in 1.25 moles of H20? 2) How many grams of He are in 9.84 x 10^22 atoms of He? 3) How many moles of
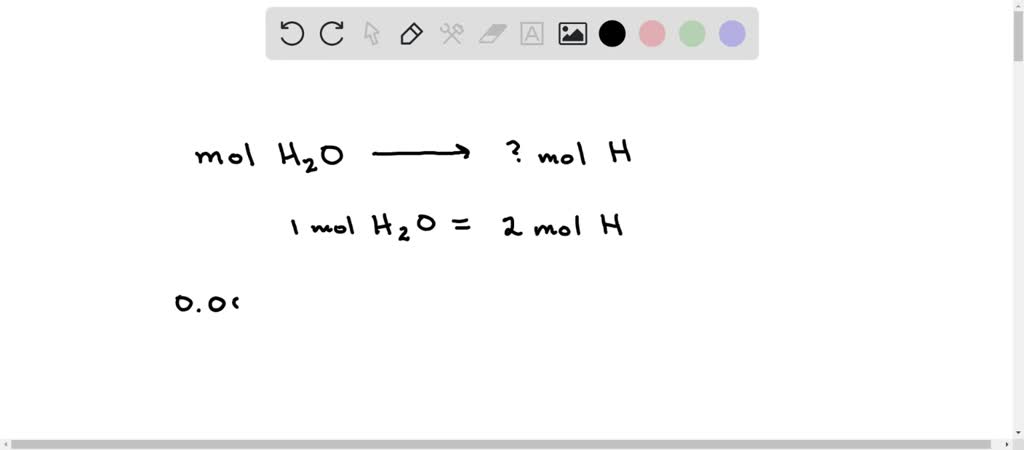
SOLVED: During the analysis, 0.00905 mol H2O is formed. Calculate the amount (mol) H in 0.00905 mol H2O.