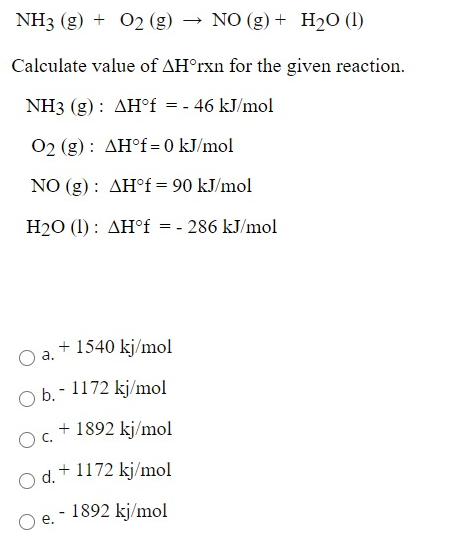
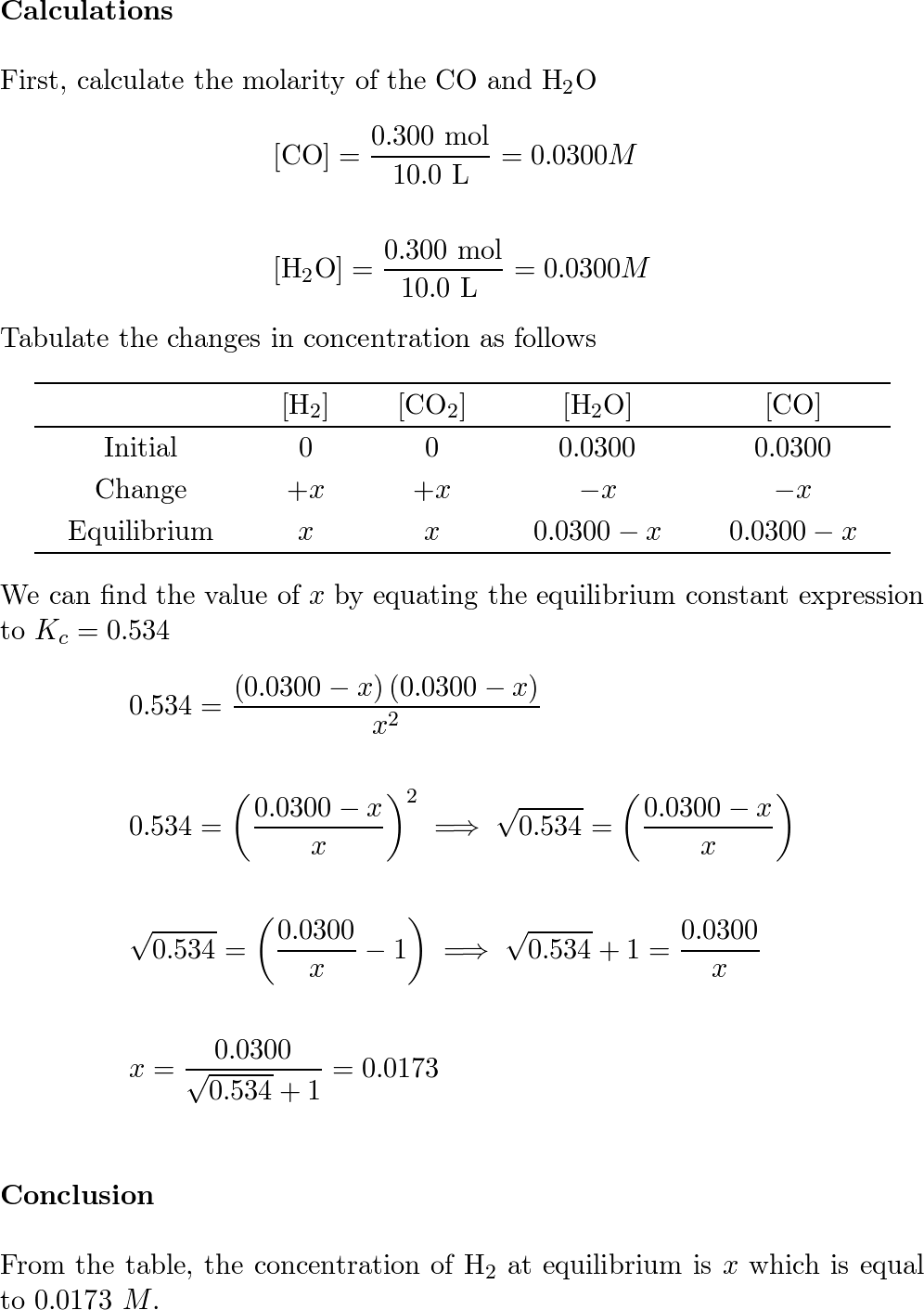For the following three reactions a, b, c, equilibrium constants are given: 1. CO (g) + H2O(g) CO2(g) + H2(g) ; K1 2. CH4(g) + H2O(g) CO(g) + 3H2(g);K2 3. CH4(g) +
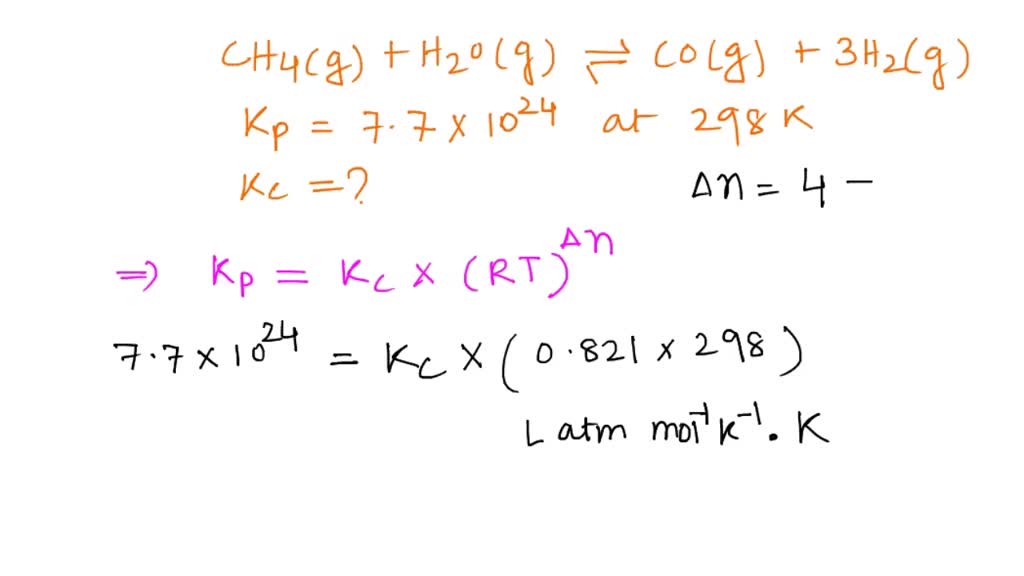
SOLVED: Consider the reaction below. H2O (g) + CH4 (g) <—> CO (g) + 3H2 (g) Kc = 4.7 at 1400 K What is Kp for this reaction at 1400 K? 6.2 x 104 4.7 8.2 x 10^8

SOLVED: When 17.07 g Al2S3 reacts with 15.54 g H2O, all the Al2S3 is used up, how many grams of excess H2O remain at the end of the reaction? Al2S3 + 6H2O —–>

Practice How many grams of H2O are produced by completely reacting 24 g of O2? 2 H2 + O2 2 H2O convert to moles convert to the desired molecule convert. - ppt download
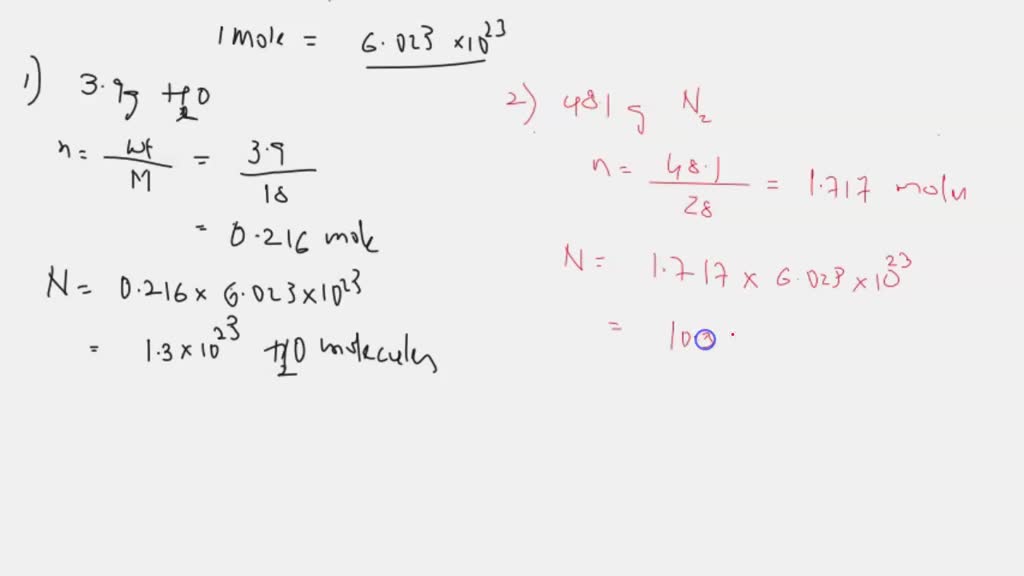
SOLVED: How many molecules are in each sample? A) 3.9g H2O Express using two significant figures. N= molecules B) 48.1g N2 Express using three significant figures. N= molecules C) 89g CCl4 Express

Practice How many grams of H2O are produced by completely reacting 24 g of O2? 2 H2 + O2 2 H2O convert to moles convert to the desired molecule convert. - ppt download