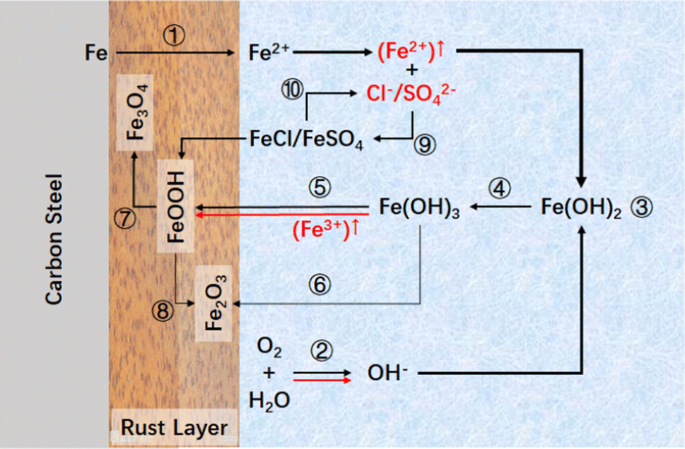
Effect of Concentrations of Fe2+ and Fe3+ on the Corrosion Behavior of Carbon Steel in Cl− and SO42− Aqueous Environments | SpringerLink
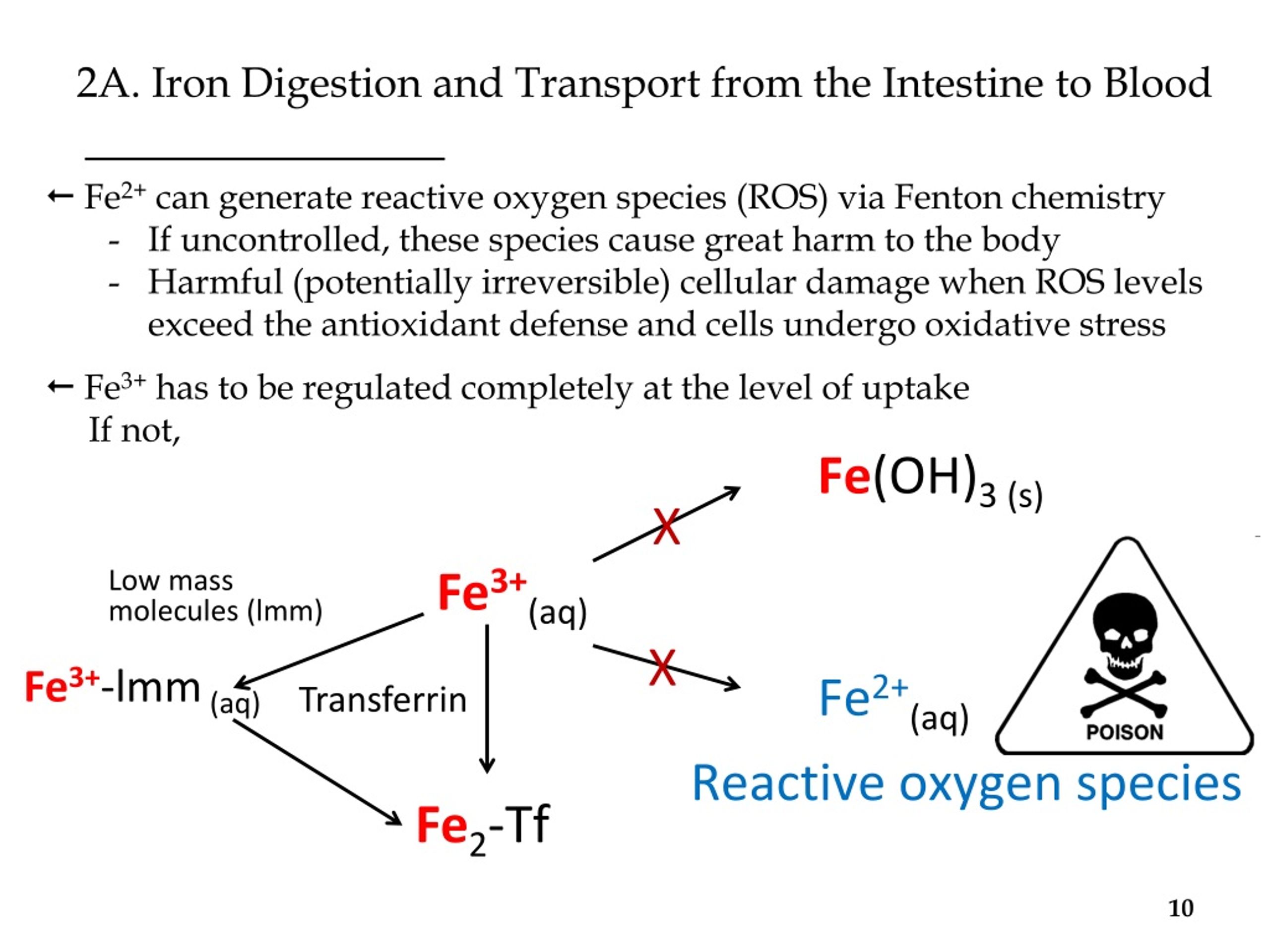
PPT - Iron (Fe 2+ /Fe 3+ ) Transport and Trafficking in Mammals Bertini et al Ch. 5 and 8 PowerPoint Presentation - ID:432903

SOLVED: Which statement is not true for the reaction: Fe2+ 5 Fe3+ Fe3+ could be referred to as an oxidizing agent in this reaction Both Fe3+ and Fe2t are called cations Fe3+

Promoting Fe3+/Fe2+ cycling under visible light by synergistic interactions between P25 and small amount of Fenton reagents - ScienceDirect

Fe2+/Fe3+ Cycling for Coupling Self‐Powered Hydrogen Evolution and Preparation of Electrode Catalysts - Chen - 2022 - Angewandte Chemie International Edition - Wiley Online Library

Color online) Energetic positions of Fe (Fe1, Fe2, and Fe3) and B (B... | Download Scientific Diagram

Double-enzymes-mediated Fe2+/Fe3+ conversion as magnetic relaxation switch for pesticide residues sensing - ScienceDirect

Enhanced electro-reduction of Fe3+ to Fe2+ by acidified carbon nanotube-modified graphite cathode and its application in a novel Fenton process for p-nitrophenol degradation - ScienceDirect

The dependence of Fe3+ and Fe2+ ions concentration in the solution from... | Download Scientific Diagram



.PNG)