
SOLVED: An ideal gas has a constant molar specific heat cv= 564 J/K/mol. Calculate the change in entropy per mole in going from the state (V1,T1)=(12 m^3,19 C) to the state (V2,T2)=(551
The CV (a) and CA (b) curves for the electrochemical oxidation of 0.5... | Download Scientific Diagram

Calculations in Chemistry To calculate the number of moles in a solid we use the following Mole Triangle g n gfm g = Mass in Grams n= Number of moles. - ppt download

SOLVED: For an ideal gas CV and Cp are different because of the workW associated with the volume change for a constant-pressure process.To explore the difference between CV and Cp for a

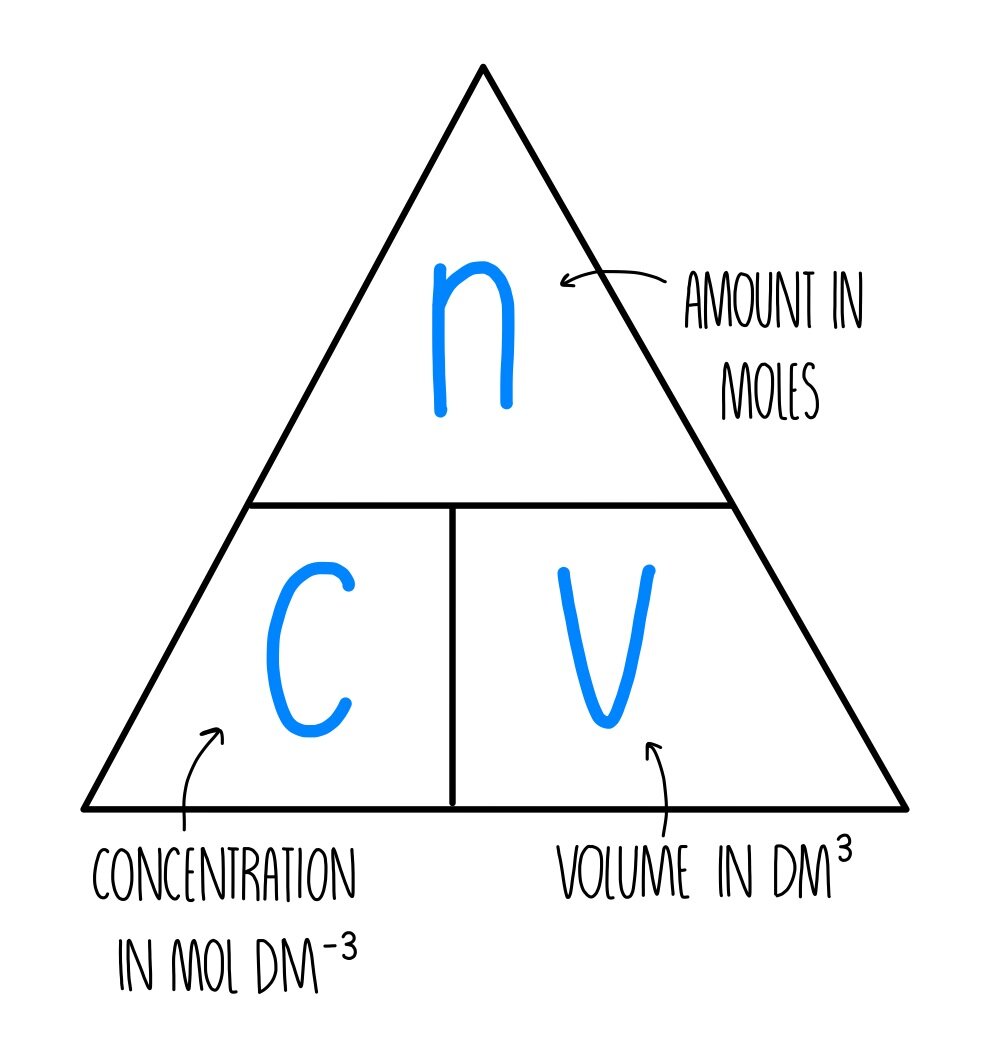
Moles and Solutions g n gfm To calculate the number of moles in a solution we use the following n CV n = number of moles C = concentatration (mol/l) V. - ppt download

For 1 mol of a triatomic ideal gas C(v) = 3R (R is universal gas constant). Fid delta (=C(p)//C(v)) for that gas.
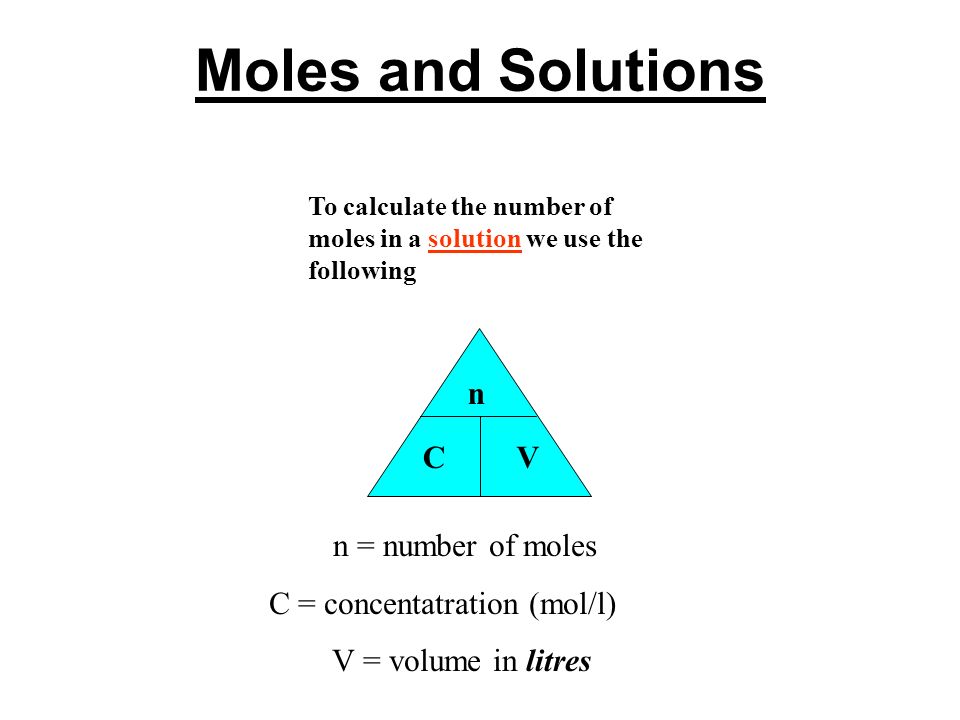
Moles and Solutions g n gfm To calculate the number of moles in a solution we use the following n CV n = number of moles C = concentatration (mol/l) V. - ppt download

SOLVED: If 2.50 mol of He gas with CV 1.5R nearly independent of T goes from 25°C and 1.00 bar to 60°C and 2.00 bar, find whichever of the following quantities can

CV curves in 1.0 mol L −1 ethanol and 1.0 mol L −1 KOH with a sweep... | Download Scientific Diagram

Calculate the value of gamma = Cp / Cv for a gaseous mixture consisting of v1 = 2.0 moles of oxygen and v2 = 3.0 moles of carbon dioxide. The gases are assumed to be ideal.



![CV for a 2 mol·dm⁻³, b 1 mol·dm⁻³ and c 0.5 mol·dm⁻³ [PMIM][Tf2N]... | Download Scientific Diagram CV for a 2 mol·dm⁻³, b 1 mol·dm⁻³ and c 0.5 mol·dm⁻³ [PMIM][Tf2N]... | Download Scientific Diagram](https://www.researchgate.net/publication/332751775/figure/fig3/AS:961858655764491@1606336382353/CV-for-a-2moldm-b-1moldm-and-c-05moldm-PMIMTf2N-solutions-at-different.png)

![N=CV, CONCENTRATION, VOLUME, NUMBER OF MOLES [Last minute revision] | Chemistry at glance - YouTube N=CV, CONCENTRATION, VOLUME, NUMBER OF MOLES [Last minute revision] | Chemistry at glance - YouTube](https://i.ytimg.com/vi/rh4IuW1fP6g/hqdefault.jpg)
