Set Of Three Chemical Containers With Acid Base And Salt With Different Ph Hcl Hydrochloric Acid Naoh Sodium Hydroxide And Nacl Sodium Chloride Stock Illustration - Download Image Now - iStock

Effect of base (NaOH) and acid (HCl) additions on changes in buffering... | Download Scientific Diagram

Vector Illustration Of Electrolytic Dissociation Molecules Break Up Into Ions Chemical Containers With Acid Base And Salt Hcl Naoh And Nacl Stock Illustration - Download Image Now - iStock

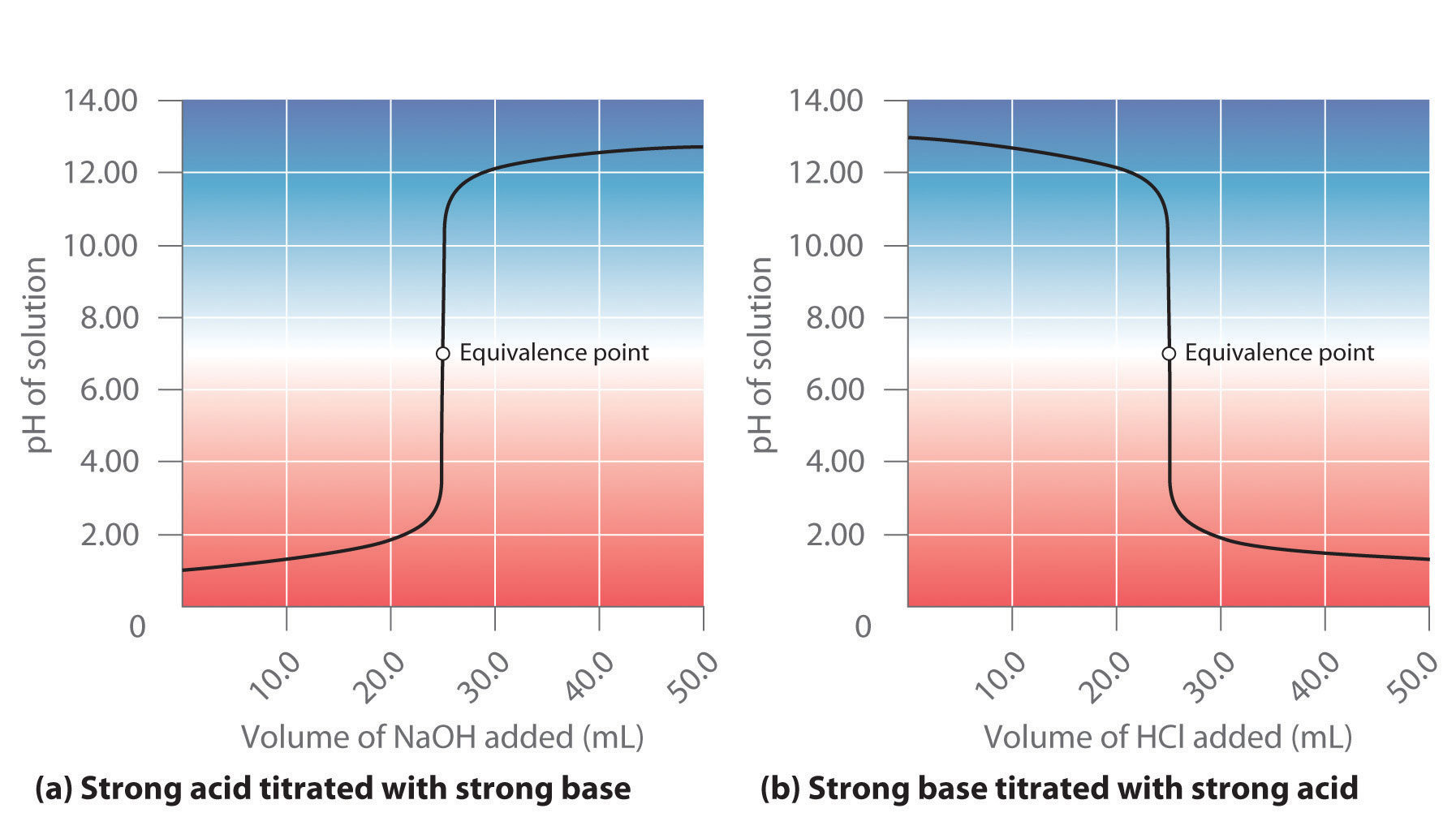
Here is an example of a titration curve, produced when a strong base is added to a strong acid. This curve shows how pH varies as 0.100 M NaOH is added to 50.0 mL of 0.100 M HCl.

Trimyristin can be hydrolyzed in base NaOH to form myristic acid. Write a balanced chemical reaction. Trimyristin should be shown in line/bond form. | Homework.Study.com
Sodium hydroxide, caustic soda, lye molecule. NaOH is highly caustic base and alkali, ionic compound. Structural chemical formula and molecule model Stock Vector Image & Art - Alamy
-in-water-01.jpg)




:max_bytes(150000):strip_icc()/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)